Targeting Spermine Oxidase to Mitigate Traumatic Brain Injury Pathology in the Aging Brain
- PMID: 40563341
- PMCID: PMC12189194
- DOI: 10.3390/antiox14060709
Targeting Spermine Oxidase to Mitigate Traumatic Brain Injury Pathology in the Aging Brain
Abstract
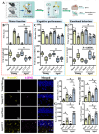
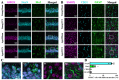
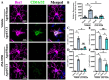
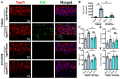
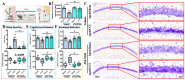
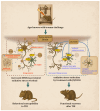
Traumatic brain injury (TBI) in the elderly is frequently associated with worsened neurological outcomes and prolonged recovery, yet the age-specific molecular mechanisms driving this vulnerability remain poorly understood. Aging is characterized by increased oxidative stress and chronic neuro-inflammation, both of which may amplify the brain's susceptibility to injury. In this study, we identify spermine oxidase (SMOX), a polyamine-catabolizing enzyme that produces reactive oxygen species, as a key mediator linking oxidative stress and neuro-inflammation to age-dependent TBI susceptibility. Using a mouse model of controlled cortical impact (CCI), we found that SMOX expression was significantly upregulated in aged brains, primarily in neurons and microglia, and this increase correlated with greater microglial activation, elevated pro-inflammatory cytokine expression, and widespread neuronal degeneration. Notably, SMOX upregulation also impaired astrocytic glutamate clearance by disrupting the membrane localization of the transporter GLT-1, contributing to excitotoxic stress. Importantly, analysis of postmortem human brain samples and transcriptomic data revealed a parallel age-related increase in SMOX expression, supporting its translational relevance. The pharmacological inhibition of SMOX with JNJ-9350 in aged mice reduced oxidative and inflammatory markers, preserved neuronal viability, and improved motor, cognitive, and emotional outcomes up to 30 days post-injury. These findings establish SMOX as a critical molecular driver of age-related vulnerability to TBI and highlight its inhibition as a promising therapeutic strategy for improving outcomes in elderly TBI patients.
Keywords: aging brain; brain injury therapeutics; neuro-inflammation; neuronal cell death; oxidative stress; spermine oxidase; traumatic brain injury.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Ma Z., He Z., Li Z., Gong R., Hui J., Weng W., Wu X., Yang C., Jiang J., Xie L., et al. Traumatic brain injury in elderly population: A global systematic review and meta-analysis of in-hospital mortality and risk factors among 2.22 million individuals. Ageing Res. Rev. 2024;99:102376. doi: 10.1016/j.arr.2024.102376. - DOI - PubMed
-
- Karaboue M.A.A., Ministeri F., Sessa F., Nannola C., Chisari M.G., Cocimano G., Di Mauro L., Salerno M., Esposito M. Traumatic Brain Injury as a Public Health Issue: Epidemiology, Prognostic Factors and Useful Data from Forensic Practice. Healthcare. 2024;12:2266. doi: 10.3390/healthcare12222266. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

