Oxidized Low-Density Lipoprotein as a Potential Target for Enhancing Immune Checkpoint Inhibitor Therapy in Microsatellite-Stable Colorectal Cancer
- PMID: 40563359
- PMCID: PMC12189509
- DOI: 10.3390/antiox14060726
Oxidized Low-Density Lipoprotein as a Potential Target for Enhancing Immune Checkpoint Inhibitor Therapy in Microsatellite-Stable Colorectal Cancer
Abstract
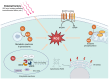
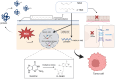
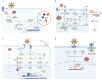
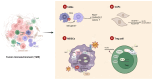
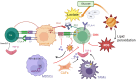
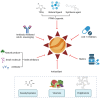
Oxidized low-density lipoprotein (oxLDL) exhibits differential expression in microsatellite-stable (MSS) and microsatellite instability-high (MSI) colorectal cancer (CRC), highlighting its potential therapeutic role in immune checkpoint inhibitor (ICI) resistance in MSS CRC. Elevated oxLDL levels in MSS CRC contribute to tumor progression and diminish ICI efficacy by modulating metabolic reprogramming and immunosuppressive mechanisms within the tumor microenvironment (TME) by activating receptors such as LOX-1 and CD36. oxLDL triggers signaling pathways, including NF-κB, PI3K/Akt, and MAPK, leading to the expansion of immunosuppressive cells like regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs), and M2 macrophages, while concurrently suppressing effector T cell functions. Additionally, oxLDL enhances oxidative stress and promotes fatty acid oxidation (FAO) and glycolytic metabolism, resulting in nutrient competition within the TME and establishing an immunosuppressive milieu, ultimately culminating in ICI resistance. This review systematically examines the disparities in oxLDL expression between MSS and MSI CRC and elucidates the molecular mechanisms through which oxLDL mediates ICI resistance. Furthermore, it explores potential therapeutic strategies targeting oxLDL, offering novel avenues to overcome immunotherapy resistance in MSS CRC.
Keywords: immune checkpoint inhibitors; immunosuppression; metabolic reprogramming; microsatellite-stable colorectal cancer; oxidative stress; oxidized low-density lipoprotein; tumor microenvironment.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Foksinski M., Rozalski R., Guz J., Ruszkowska B., Sztukowska P., Piwowarski M., Klungland A., Olinski R. Urinary excretion of DNA repair products correlates with metabolic rates as well as with maximum life spans of different mammalian species. Free Radic. Biol. Med. 2004;37:1449–1454. doi: 10.1016/j.freeradbiomed.2004.07.014. - DOI - PubMed
-
- Wei S.C., Duffy C.R., Allison J.P. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018;8:1069–1086. doi: 10.1158/2159-8290.CD-18-0367. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

