Restoring Glutathione Homeostasis in Glycation-Related Eye Diseases: Mechanistic Insights and Therapeutic Interventions Beyond VEGF Inhibition
- PMID: 40563363
- PMCID: PMC12189886
- DOI: 10.3390/antiox14060731
Restoring Glutathione Homeostasis in Glycation-Related Eye Diseases: Mechanistic Insights and Therapeutic Interventions Beyond VEGF Inhibition
Abstract
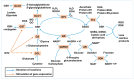
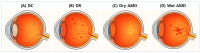
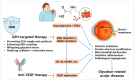
Advanced glycation end-products (AGEs) and oxidative stress are recognized as central contributors to the pathogenesis of age-related or diabetic cataracts, diabetic retinopathy (DR), and age-related macular degeneration (AMD). These glycation-related diseases are characterized by impaired redox balance and decreased glutathione (GSH) levels. This review aims to examine the mechanistic links between AGEs and GSH depletion across ocular tissues by integrating in vitro, ex vivo, in vivo, and clinical studies relevant to this topic. The multiple levels of evidence highlight GSH homeostasis as both a biomarker and therapeutic target in glycation-related ocular disorders. Therapeutic strategies aimed at restoring GSH homeostasis under glycation stress are categorized into four mechanistic domains: (I) promoting GSH supply and synthesis, (II) enhancing GSH recycling, (III) mitigating glycation stress, and (IV) reducing oxidative and nitrosative stress. Most of these strategies have been explored via different approaches, and experimental findings with various interventions have shown promise in restoring GSH balance and mitigating AGE-induced damage. A pathological link between GSH depletion and vascular endothelial growth factor (VEGF) overexpression is observed in DR and wet AMD. GSH-centered interventions act upstream to modulate redox homeostasis while anti-VEGF therapies target downstream angiogenesis. This study supports the rationale for a dual-targeting strategy that combines redox-based interventions with VEGF inhibition in glycation-related ocular diseases.
Keywords: advanced glycation end-products; age-related macular degeneration; aldose reductase inhibitors; cataracts; diabetic retinopathy; glutathione; glyoxalase; oxidative stress; redox homeostasis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Lam N.V. Adult Eye Conditions: Diabetic Retinopathy and Age-Related Macular Degeneration. FP Essent. 2022;519:24–28. - PubMed
-
- Castro-Castaneda C.R., Altamirano-Lamarque F., Ortega-Macias A.G., Santa Cruz-Pavlovich F.J., Gonzalez-De la Rosa A., Armendariz-Borunda J., Santos A., Navarro-Partida J. Nutraceuticals: A Promising Therapeutic Approach in Ophthalmology. Nutrients. 2022;14:5014. doi: 10.3390/nu14235014. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

