Redox Imbalance Is Associated with Neuronal Apoptosis in the Cortex of Neonates Gestated Under Chronic Hypoxia
- PMID: 40563368
- PMCID: PMC12189337
- DOI: 10.3390/antiox14060736
Redox Imbalance Is Associated with Neuronal Apoptosis in the Cortex of Neonates Gestated Under Chronic Hypoxia
Abstract
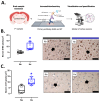
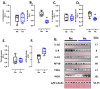
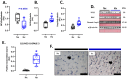
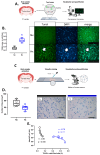
Gestational chronic hypoxia impacts prenatal development, leading to fetal growth restriction (FGR), defined as the fetus's failure to reach its genetic growth potential. Postnatal hypoxia in the cerebral tissue can induce a redox imbalance and mitochondrial dysfunction, consequently increasing neuronal death. However, these data cannot necessarily be extrapolated to prenatal hypoxia. In this regard, this study aims to describe the effect of gestational hypoxia on redox balance and apoptosis cell death mechanisms in the prefrontal cortex of guinea pigs. Ten Guinea pig (Cavia porcellus) pregnant dams were utilized in this study; five gestated in normoxia (Nx; three newborn males, and two females) and five gestated under chronic hypobaric hypoxia (Hx; two newborn males, and three females). We monitored the pregnancies by ultrasound examinations from gestational days 20 to 65 (term ~ 70). At birth, pups were euthanized, and the fetal brain was collected for cellular redox measurement, mitochondrial enzyme expression, and apoptosis assay. Gestation under hypoxia induced an imbalance in the expression of anti- and pro-oxidant enzymes, resulting in increased oxidative stress. Additionally, a decrease in cytochrome I and III expression and neuronal density in the neonatal prefrontal cortex was observed. Finally, DNA fragmentation was increased by the TUNEL assay in the brain tissue of newborns gestated under chronic hypoxia. Our findings demonstrate the association of gestational hypoxia with oxidative stress and neuronal death in newborns, which may predispose to neuronal dysfunction in adulthood.
Keywords: intrauterine chronic hypoxia; mitochondrial dysfunction; neuron death; oxidative stress.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

