Molecular and Biochemical Mechanisms of Cardiomyopathy Development Following Prenatal Hypoxia-Focus on the NO System
- PMID: 40563375
- PMCID: PMC12189570
- DOI: 10.3390/antiox14060743
Molecular and Biochemical Mechanisms of Cardiomyopathy Development Following Prenatal Hypoxia-Focus on the NO System
Abstract
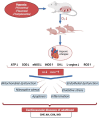
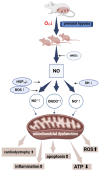
Prenatal hypoxia (PH) adversely affects the development of the fetal heart, contributing to persistent cardiovascular impairments in postnatal life. A key component in regulating cardiac physiology is the nitric oxide (NO) system, which influences vascular tone, myocardial contractility, and endothelial integrity during development. Exposure to PH disrupts NO-related signaling pathways, leading to endothelial dysfunction, mitochondrial damage, and an escalation of oxidative stress-all of which exacerbate cardiac injury and trigger cardiomyocyte apoptosis. The excessive generation of reactive nitrogen species drives nitrosative stress, thereby intensifying inflammatory processes and cellular injury. In addition, the interplay between NO and hypoxia-inducible factor (HIF) shapes adaptive responses to PH. NO also modulates the synthesis of heat shock protein 70 (HSP70), a critical factor in cellular defense against stress. This review emphasizes the involvement of NO in cardiovascular injury caused by PH and examines the cardioprotective potential of NO modulators-Angiolin, Thiotriazoline, Mildronate, and L-arginine-as prospective therapeutic agents. These agents reduce oxidative stress, enhance endothelial performance, and alleviate the detrimental effects of PH on the heart, offering potential new strategies to prevent cardiovascular disorders in offspring subjected to prenatal hypoxia.
Keywords: HSP70; NO modulators; cardiomyocyte apoptosis; cardiomyopathy; endothelial dysfunction; mitochondrial dysfunction; nitrosative stress; oxidative stress; prenatal hypoxia.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Narohan M.V., Bazhenova L.K., Kapranova E.I., Melnikova E.V., Belousova N.A. Posthypoxic Dysfunction of the Cardiovascular System in Newborns. Curr. Issues Pediatr. 2007;6:42–46. (In Ukranian)
Publication types
LinkOut - more resources
Full Text Sources

