Prebiotic Oligosaccharides in Skin Health: Benefits, Mechanisms, and Cosmetic Applications
- PMID: 40563386
- PMCID: PMC12189978
- DOI: 10.3390/antiox14060754
Prebiotic Oligosaccharides in Skin Health: Benefits, Mechanisms, and Cosmetic Applications
Abstract
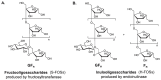
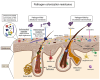
Prebiotic oligosaccharides have attracted significant interest in dermatology and skin health due to their ability to modulate the skin microbiome and microbiota-host interactions. This review offers a novel dual perspective, systematically examining the benefits of both oral intake and topical application of prebiotic oligosaccharides, including well-established prebiotics (e.g., human milk oligosaccharides, galacto- and fructo-oligosaccharides) and emerging prebiotic candidates (e.g., gluco-oligosaccharides, chitosan-oligosaccharides, agaro-oligosaccharides). First, cutting-edge synthetic processes for producing diverse oligosaccharides and their structural chemistry are introduced. Then, we discuss in vitro studies demonstrating their efficacy in promoting skin commensals, inhibiting pathogens, and conferring protective effects, such as antioxidant, anti-inflammatory, anti-melanogenic, and wound-healing properties. Furthermore, we emphasize in vivo animal studies and clinical trials revealing that prebiotic oligosaccharides, administered orally or topically, alleviate atopic dermatitis, enhance skin hydration, attenuate acne, and protect against photo-aging by modulating skin-gut microbiota and immune responses. Mechanistically, we integrate genetic and molecular insights to elucidate how oligosaccharides mediate these benefits, including gut-skin axis crosstalk, immune regulation, and microbial metabolite signaling. Finally, we highlight current commercial applications of oligosaccharides in cosmetic formulations while addressing scientific and practical challenges, such as structure-function relationships, clinical scalability, and regulatory considerations. This review bridges mechanistic understanding with practical applications, offering a comprehensive resource for advancing prebiotic oligosaccharides-based skincare therapies.
Keywords: commercial cosmetics; non-digestible oligosaccharides; pathogen inhibition; skin barrier function; skin diseases.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures








References
-
- Demessant-Flavigny A.-L., Connétable S., Kerob D., Moreau M., Aguilar L., Wollenberg A. Skin microbiome dysbiosis and the role of Staphylococcus aureus in atopic dermatitis in adults and children: A narrative review. J. Eur. Acad. Dermatol. Venereol. 2023;37:3–17. doi: 10.1111/jdv.19125. - DOI - PubMed
Publication types
Grants and funding
- MZGC20240028/Sichuan Science and Technology Innovation Seedling Project
- 22208111/National Natural Science Foundation of China
- 22378077/National Natural Science Foundation of China
- 2023A1515010064/Guangdong Basic and Applied Basic Research Foundation
- 2023QNRC001/Young Elite Scientists Sponsorship Program by CAST
LinkOut - more resources
Full Text Sources

