Enhanced Innate Immunity Mediated by IL-36α in Atopic Dermatitis and Differences in Cytokine Profiles of Lymphocytes in the Skin and Draining Lymph Nodes
- PMID: 40563457
- PMCID: PMC12190923
- DOI: 10.3390/biom15060817
Enhanced Innate Immunity Mediated by IL-36α in Atopic Dermatitis and Differences in Cytokine Profiles of Lymphocytes in the Skin and Draining Lymph Nodes
Abstract
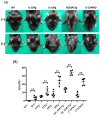
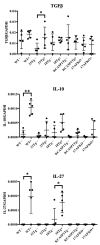
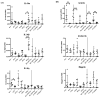
(1) Background: The IL-36 cytokines have been identified as key contributors to pustular psoriasis, and their inhibitor is already in clinical use. However, few studies have explored them in atopic dermatitis. (2) Methods: The role of IL-36α was investigated in various atopic dermatitis models using wild-type, keratin 14-specific IL-33 transgenic, IL-18 transgenic, caspase-1 transgenic, and caspase-1 transgenic mice with IL-17AF deletion, reflecting diverse aspects of human skin inflammation. IL-36α was administered subcutaneously in five doses on alternate days across the five strains to examine cellular infiltration patterns and cytokine expression levels. (3) Results: The skin phenotype was exacerbated, accompanied by worsening edema and skin thickness in all mouse groups upon IL-36α administration. An increase in infiltrating cells was observed among innate immune cells, while lymphocyte counts, including T cells and innate lymphoid cells, did not rise. Additionally, anti-inflammatory cytokines were induced simultaneously with inflammatory cytokines and downstream cytokines of IL-36α as well. Infiltrating lymphocytes in the skin displayed a distinct Type 2 cytokine-dominant profile for innate lymphoid cells and a Type 3 cytokine-dominant profile for T helper cells and γδ T cells, contrasting with the Type 1-dominant cell profile in draining lymph nodes. Type 1, Type 2, and Type 3 cytokine dominance patterns were not affected by the administration of IL-36α. (4) Conclusions: IL-36α triggers inflammatory responses in atopic dermatitis by activating innate immunity. The infiltrating lymphocytes in the skin have different cytokine production profiles between innate lymphoid cells and T cells, as well as different patterns of cytokine production in their draining lymph nodes.
Keywords: alarmin; atopic dermatitis; inflammation; interleukin-36α; psoriasis.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of this study; in the collection, analysis, or interpretation of data; in the writing of this manuscript; or in the decision to publish these results.
Figures


Similar articles
-
IL-36/IL-36R signaling promotes CD4+ T cell-dependent colitis via pro-inflammatory cytokine production.Front Immunol. 2025 Jun 26;16:1604332. doi: 10.3389/fimmu.2025.1604332. eCollection 2025. Front Immunol. 2025. PMID: 40642091 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Oncostatin M enhances the lengthening of sensory nerves and skin hypersensitivity.Front Immunol. 2025 Jul 3;16:1571120. doi: 10.3389/fimmu.2025.1571120. eCollection 2025. Front Immunol. 2025. PMID: 40677708 Free PMC article.
-
Towards a patient-centred definition for atopic dermatitis flare: a qualitative study of adults with atopic dermatitis.Br J Dermatol. 2024 Jun 20;191(1):82-91. doi: 10.1093/bjd/ljae037. Br J Dermatol. 2024. PMID: 38287887
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
References
-
- Kato S., Matsushima Y., Mizutani K., Kawakita F., Fujimoto M., Okada K., Kondo M., Habe K., Suzuki H., Mizutani H., et al. The Stenosis of Cerebral Arteries and Impaired Brain Glucose Uptake by Long-Lasting Inflammatory Cytokine Release from Dermatitis Is Rescued by Anti-IL-1 Therapy. J. Investig. Dermatol. 2018;138:2280–2283. doi: 10.1016/j.jid.2018.04.016. - DOI - PubMed
-
- Nakanishi T., Iida S., Maruyama J., Urushima H., Ichishi M., Matsushima Y., Mizutani K., Nakayama Y., Sugioka K., Nishimura M., et al. Arteriosclerosis Derived from Cutaneous Inflammation Is Ameliorated by the Deletion of IL-17A and IL-17F. Int. J. Mol. Sci. 2023;24:5434. doi: 10.3390/ijms24065434. - DOI - PMC - PubMed
-
- Yamanaka K., Nakanishi T., Saito H., Maruyama J., Isoda K., Yokochi A., Imanaka-Yoshida K., Tsuda K., Kakeda M., Okamoto R., et al. Persistent release of IL-1s from skin is associated with systemic cardio-vascular disease, emaciation and systemic amyloidosis: The potential of anti-IL-1 therapy for systemic inflammatory diseases. PLoS ONE. 2014;9:e104479. doi: 10.1371/journal.pone.0104479. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

