The Role of LAIR1 as a Regulatory Receptor of Antitumor Immune Cell Responses and Tumor Cell Growth and Expansion
- PMID: 40563506
- PMCID: PMC12191340
- DOI: 10.3390/biom15060866
The Role of LAIR1 as a Regulatory Receptor of Antitumor Immune Cell Responses and Tumor Cell Growth and Expansion
Abstract
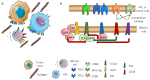
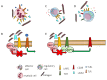
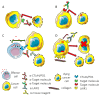
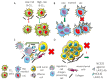
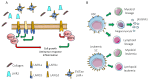
It is becoming evident that the therapeutic effect of reawakening the immune response is to limit tumor cell growth and expansion. The use of immune checkpoint inhibitors, like blocking antibodies against programmed cell death receptor (PD) 1 and/or cytotoxic T lymphocyte antigen (CTLA) 4 alone or in combination with other drugs, has led to unexpected positive results in some tumors but not all. Several other molecules inhibiting lymphocyte antitumor effector subsets have been discovered in the last 30 years. Herein, we focus on the leukocyte-associated immunoglobulin (Ig)-like receptor 1 (LAIR1/CD305). LAIR1 represents a typical immunoregulatory molecule expressed on almost all leukocytes, unlike other regulatory receptors expressed on discrete leukocyte subsets. It bears two immunoreceptor tyrosine-based inhibitory motifs (ITIMs) in the intracytoplasmic protein domain involved in the downregulation of signals mediated by activating receptors. LAIR1 binds to several ligands, such as collagen I and III, complement component 1Q, surfactant protein D, adiponectin, and repetitive interspersed families of polypeptides expressed by erythrocytes infected with Plasmodium malariae. This would suggest LAIR1 involvement in several cell-to-cell interactions and possibly in metabolic regulation. The presence of both cellular and soluble forms of LAIR would indicate a fine regulation of the immunoregulatory activity, as happens for the soluble/exosome-associated forms of PD1 and CTLA4 molecules. As a consequence, LAIR1 appears to play a role in some autoimmune diseases and the immune response against tumor cells. The finding of LAIR1 expression on hematological malignancies, but also on some solid tumors, could open a rationale for the targeting of this molecule to treat neoplasia, either alone or in combination with other therapeutic options.
Keywords: LAIR1; immune checkpoint; immune regulation; immunotherapy; inhibitory receptors.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Antibody tests for identification of current and past infection with SARS-CoV-2.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD013652. doi: 10.1002/14651858.CD013652.pub2. Cochrane Database Syst Rev. 2022. PMID: 36394900 Free PMC article.
-
Combined programmed cell death protein 1 and cytotoxic T-lymphocyte associated protein 4 blockade in an international cohort of patients with acral lentiginous melanoma.Br J Dermatol. 2025 Jan 24;192(2):316-326. doi: 10.1093/bjd/ljae401. Br J Dermatol. 2025. PMID: 39438074
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

