Targeting Cellular Senescence to Enhance Human Endometrial Stromal Cell Decidualization and Inhibit Their Migration
- PMID: 40563513
- PMCID: PMC12191167
- DOI: 10.3390/biom15060873
Targeting Cellular Senescence to Enhance Human Endometrial Stromal Cell Decidualization and Inhibit Their Migration
Abstract
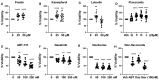
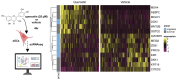
Cellular senescence leads to stable cell cycle arrest and an inflammatory senescence-associated secretory phenotype that varies with stressor and cell type. To mitigate these effects and improve health, senotherapeutics (e.g., senolytics and senomorphics) have been developed. Senescent-like endometrial stromal cells (eSCs) lining the uterus of patients with endometriosis and infertility are proposed to impair decidualization, a differentiation process required for uterine receptivity in humans. Quercetin, a natural flavonoid senolytic, dramatically improves decidualization and reduces endometriosis in rodent models. However, little is known about the comparative effects of various senotherapeutics on eSCs. Using menstrual effluent-derived eSCs, we evaluated the effects of flavonoid and non-flavonoid compounds on eSC functions associated with endometriosis, aiming to identify optimal senotherapeutics for future clinical trials. Among flavonoids tested, all senolytics (quercetin, fisetin, and luteolin) and kaempferol, a senomorphic, significantly improved decidualization without cytotoxicity. Although non-flavonoids exhibited notable cytotoxicity, dasatinib, but neither ABT-737 nor navitoclax, enhanced decidualization. Flavonoid senotherapeutics and dasatinib significantly inhibited eSC migration. Mechanistic studies revealed that all flavonoids and dasatinib suppressed AKT phosphorylation and upregulated p53 expression. Notably, only quercetin and fisetin reduced ERK1/2 phosphorylation. Furthermore, flavonoid-senolytics and dasatinib consistently eliminated senescent eSCs. These findings support future studies to assess the therapeutic potential of in vivo supplementation with flavonoid senolytics on eSC function using menstrual effluent.
Keywords: endometriosis; flavonoids; infertility; menstrual effluent; quercetin; senescence; senolytic; senomorphic.
Conflict of interest statement
A.G.B., L.F. and D.M.E. are co-founders of Senesys Bio, delivering a new generation of targeted senolytics.
Figures




Similar articles
-
Quercetin enhances decidualization through AKT-ERK-p53 signaling and supports a role for senescence in endometriosis.Reprod Biol Endocrinol. 2024 Aug 8;22(1):100. doi: 10.1186/s12958-024-01265-z. Reprod Biol Endocrinol. 2024. PMID: 39118090 Free PMC article.
-
Apoptotic priming in senescence predicts specific senolysis by quantitative analysis of mitochondrial dependencies.Cell Death Differ. 2025 May;32(5):802-817. doi: 10.1038/s41418-024-01431-1. Epub 2025 Jan 6. Cell Death Differ. 2025. PMID: 39762561
-
The senolytic cocktail, dasatinib and quercetin, impacts the chromatin structure of both young and senescent vascular smooth muscle cells.Geroscience. 2025 Jun;47(3):3907-3925. doi: 10.1007/s11357-024-01504-6. Epub 2025 Jan 20. Geroscience. 2025. PMID: 39828770 Free PMC article.
-
Targeting Senescence: A Review of Senolytics and Senomorphics in Anti-Aging Interventions.Biomolecules. 2025 Jun 13;15(6):860. doi: 10.3390/biom15060860. Biomolecules. 2025. PMID: 40563501 Free PMC article. Review.
-
Cellular senescence and senotherapeutics in cardiovascular diseases.Adv Pharmacol. 2025;104:313-349. doi: 10.1016/bs.apha.2025.01.019. Epub 2025 Feb 24. Adv Pharmacol. 2025. PMID: 40716934 Review.
References
-
- Reyes A., Ortiz G., Duarte L.F., Fernandez C., Hernandez-Armengol R., Palacios P.A., Prado Y., Andrade C.A., Rodriguez-Guilarte L., Kalergis A.M., et al. Contribution of viral and bacterial infections to senescence and immunosenescence. Front. Cell Infect. Microbiol. 2023;13:1229098. doi: 10.3389/fcimb.2023.1229098. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

