Trastuzumab Deruxtecan in Previously Treated HER2-Low Metastatic Breast Cancer: Real-World Multicentric Study in the Portuguese Population
- PMID: 40563562
- PMCID: PMC12190700
- DOI: 10.3390/cancers17121911
Trastuzumab Deruxtecan in Previously Treated HER2-Low Metastatic Breast Cancer: Real-World Multicentric Study in the Portuguese Population
Abstract
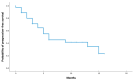
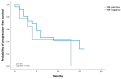
Background/Objectives: Breast cancer is the most common malignant neoplasm in women and the leading cause of cancer-related death. Approximately 50% of HER2-negative breast cancers exhibit low expression of this protein (HER2-low). Trastuzumab deruxtecan (T-DXd) is an antibody-drug conjugate targeting the HER2 receptor which has shown benefit in patients with HER2-low metastatic breast cancer in the DESTINY-Breast04 study. However, few data are available on its efficacy in real-world practice. Methods: We conducted a retrospective multicenter national study (eight centers) including patients with advanced HER2-low breast cancer (immunohistochemistry 1+ or 2+/ in situ hybridization negative) who started T-DXd treatment between January 2022 and March 2024. Patients had received at least one previous line of treatment. The primary endpoint was real-world progression-free survival (rwPFS) in patients with metastatic HER2-low breast cancer treated with T-DXd. The secondary endpoints were real-world overall survival (OS) and objective response rate (ORR). Results: The study included 35 patients (34 female and 1 male patient), with a median age of 54 years at the start of T-DXd. All patients had an ECOG-PS 0-1, and 26 patients (74%) had hormone receptor (HR)-positive disease. The median number of prior lines of treatment was 4 [1-7], and 23 patients (65.8%) had metastases in three or more sites. With a median follow-up of 7.8 months, rwPFS was 6 months (95% CI, 2.3-9.7), and OS was 15 months (95% CI, 4.7-25.3). In HR-positive patients, the median rwPFS was 6 months (95% CI, 1.2-10.7), compared to 4 months (95% CI, 2.1-5.9) in HR-negative patients. The overall ORR was 52.9%. Adverse events of grade 3 or higher were neutropenia (2.9%) and fatigue (2.9%). Conclusions: This study provides real-world data on T-DXd in the treatment of advanced HER2-low breast cancer. It is noteworthy that the population was heavily pre-treated and had a higher proportion of HR-negative patients, which may explain the lower efficacy compared to the DESTINY-Breast04 study.
Keywords: HER2-low; breast cancer; trastuzumab deruxtecan.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous