The RXR Agonist MSU-42011 Reduces Tumor Burden in a Murine Preclinical NF1-Deficient Model
- PMID: 40563570
- PMCID: PMC12190937
- DOI: 10.3390/cancers17121920
The RXR Agonist MSU-42011 Reduces Tumor Burden in a Murine Preclinical NF1-Deficient Model
Abstract
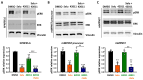
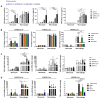
Background/Objectives: Neurofibromatosis type 1 (NF1) is a prevalent inherited disorder, with approximately 50% of affected individuals developing plexiform neurofibromas (PNFs), which can progress to highly aggressive malignant peripheral nerve sheath tumors (MPNSTs). While selumetinib is FDA-approved for PNFs, its efficacy in MPNSTs is limited and associated with dose-limiting toxicities. NF1 deficiency drives tumorigenesis and alters immune dynamics via RAS hyperactivation. Given the substantial macrophage infiltration in NF1 lesions and its association with disease progression, we hypothesized that targeting tumor-promoting immune cells with the retinoid X receptor (RXR) agonist MSU-42011 could be an alternative therapeutic strategy, as it has shown promise in KRAS-driven cancers by decreasing pERK levels and reducing tumor-promoting immune cells. Methods: We examined the effects of MSU-42011 and selumetinib, alone and in combination, on NF1-deficient cells and in a syngeneic MPNST model. Results: In vivo, the combination of MSU-42011 and selumetinib significantly reduced tumor growth, pERK levels, and tumor-promoting macrophages and increased activated CD8+ T cells in syngeneic MPNST models. In NF1-deficient cells, MSU-42011 or selumetinib reduced pERK levels, with combination treatment achieving greater reductions. Conditioned media (CM) from NF1-deficient cells increased the protein and mRNA levels of several cytokines and chemokines in human THP1 cells and bone marrow-derived macrophages (BMDMs). MSU-42011 and selumetinib, alone or in combination, partially reversed this induction. Conclusions: These findings suggest RXR agonists may have therapeutic potential against NF1, and their combination with MEK inhibitors could represent a promising strategy for NF1-associated tumors. Further studies are needed to validate these results and assess their translational relevance.
Keywords: malignant peripheral nerve sheath tumors (MPNSTs); neurofibromatosis type 1; plexiform neurofibromas (PNFs); retinoid X receptor (RXR) agonist; selumetinib.
Conflict of interest statement
A.S.L., B.A., E.E. and K.T.L. are named inventors on a patent filed on the novel RXR agonist and owned by MSU. The other authors have no potential conflicts of interest.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

