DCIS Progression and the Tumor Microenvironment: Molecular Insights and Prognostic Challenges
- PMID: 40563575
- PMCID: PMC12190921
- DOI: 10.3390/cancers17121925
DCIS Progression and the Tumor Microenvironment: Molecular Insights and Prognostic Challenges
Abstract
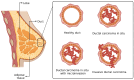
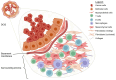
Ductal carcinoma in situ (DCIS) is the most common form of non-invasive breast cancer and a recognized precursor to invasive ductal carcinoma (IDC). Although DCIS itself is confined to the milk duct and not immediately life-threatening, its potential for progression to invasive disease necessitates careful clinical management. The increased detection of DCIS due to advancements in imaging and widespread screening programs has raised critical questions regarding its classification, prognosis, and optimal treatment strategies. While most cases exhibit indolent behavior, others harbor molecular characteristics that drive malignant transformation. A key challenge lies in distinguishing low-risk DCIS, which may never progress, from aggressive cases requiring intervention. Tumor microenvironment dynamics, immune cell infiltration, and molecular alterations, including hormone receptor (HR) status, human epidermal growth factor 2 (HER2) expression, and genetic mutations, play crucial roles in determining disease trajectory. This review explores the biological and molecular mechanisms underlying DCIS progression, with an emphasis on myoepithelial cells, tumor-infiltrating lymphocytes, and microenvironmental factors. By integrating recent findings, this article aims to refine risk stratification approaches and guide future strategies for personalized DCIS management. Improved prognostic biomarkers and targeted therapeutic interventions could help optimize treatment decisions, balancing the need for effective cancer prevention while minimizing overtreatment in low-risk patients.
Keywords: DCIS progression; ductal carcinoma in situ; tumor microenvironment.
Conflict of interest statement
The author declares no conflicts of interest.
Figures

References
-
- Mathelin C., Lodi M., Alghamdi K., Arboleda-Osorio B., Avisar E., Anyanwu S., Boubnider M., Costa M.M., Elder E., Elonge T., et al. The Senologic International Society Survey on Ductal Carcinoma In Situ: Present and Future. Eur. J. Breast Health. 2022;18:205–221. doi: 10.4274/ejbh.galenos.2022.2022-4-3. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

