Next-Generation CAR-T and TCR-T Cell Therapies for Solid Tumors: Innovations, Challenges, and Global Development Trends
- PMID: 40563595
- PMCID: PMC12191048
- DOI: 10.3390/cancers17121945
Next-Generation CAR-T and TCR-T Cell Therapies for Solid Tumors: Innovations, Challenges, and Global Development Trends
Abstract
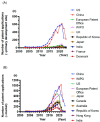
Chimeric antigen receptor (CAR)-T and T-cell receptor (TCR)-engineered T-cell (TCR-T) therapies have revolutionized the treatment of hematological malignancies; however, their application to solid tumors remains a formidable challenge. The immunosuppressive tumor microenvironment, antigen heterogeneity, and manufacturing complexity limit the clinical efficacy and scalability of these treatment modalities. This review provides a comprehensive analysis of the current clinical development strategies for CAR-T and TCR-T cell therapies for solid tumors. Herein, we discuss recent breakthroughs and highlight the potential of TCR-T cell therapy. Furthermore, innovative approaches for enhancing CAR-T cell function in solid tumors (e.g., in vivo engineering; induced pluripotent stem cell-derived allogeneic CAR-T cells; armored CAR constructs; dual-antigen targeting; and combination regimens with checkpoint inhibitors, chemotherapy, radiotherapy, and oncolytic viruses) are explored. We also present trends in global patent activity, revealing a marked acceleration in CAR-T- and TCR-T-related innovations, with the United States and China leading with respect to application volumes. This field is increasingly characterized by multidisciplinary collaborations between academia and industry, driving the development of next-generation platforms, including messenger RNA-based and off-the-shelf cell therapies. Although no CAR-T product has been approved for solid tumors, these findings underscore the accelerating momentum and translational promise of adoptive cell therapies. Addressing the unique biological and logistical challenges of solid tumors is essential for realizing the full potential of these transformative immunotherapies.
Keywords: CAR-T cell therapy; TCR-T cell therapy; adoptive cell therapy; intellectual property; patent landscape; solid tumors.
Conflict of interest statement
T.S. and Y.K. have nothing to declare. T.K. has received honoraria from Chugai Pharma, Sysmex, and AstraZeneca and served in a consulting or advisory role for Amgen. Research funding has been received by the author’s institution from the following companies: PACT Pharma, Novartis, Lilly, Takeda, Daiichi Sankyo RD Novare, Chugai Pharma, Zymeworks, Pfizer, Janssen Pharma, Boehringer Ingelheim, and AstraZeneca. T.N. holds patents with OncoTherapy Science, Inc., Noile-Immune Biotech Inc., Thyas Co., Ltd., Resonac Corporation, MEDINET Co., Ltd., and Takara Bio Inc., holds stock in Noile-Immune Biotech Inc., holds stock options and has received grants or contracts from Logomix Inc. and Optieum Biotechnologies Inc., has received grants or contracts from BrightPath Biotherapeutics Co., Ltd., Thyas Co., Ltd., ONO PHARMACEUTICAL CO., LTD., Resonac Corporation, MEDINET Co., Ltd., NapaJen Pharma Inc., Heartseed Inc., Takara Bio Inc., DAICEL CORPORATION, NA Vaccine Institute CO., LTD., and MaxCyte, Inc.
Figures

Similar articles
-
CAR T-cell immunotherapy as the next horizon in cancer eradication: current landscape, challenges, and future directions.Med Oncol. 2025 Aug 6;42(9):410. doi: 10.1007/s12032-025-02957-1. Med Oncol. 2025. PMID: 40770153 Review.
-
Current Advances and Challenges in CAR-T Therapy for Hematological and Solid Tumors.Immunotargets Ther. 2025 Jun 27;14:655-680. doi: 10.2147/ITT.S519616. eCollection 2025. Immunotargets Ther. 2025. PMID: 40599347 Free PMC article. Review.
-
Transduction of γδ T cells with Baboon envelope pseudotyped lentiviral vector encoding chimeric antigen receptors for translational and clinical applications.Front Immunol. 2025 Jun 6;16:1548630. doi: 10.3389/fimmu.2025.1548630. eCollection 2025. Front Immunol. 2025. PMID: 40547029 Free PMC article.
-
Engineering allorejection-resistant CAR-NKT cells from hematopoietic stem cells for off-the-shelf cancer immunotherapy.Mol Ther. 2024 Jun 5;32(6):1849-1874. doi: 10.1016/j.ymthe.2024.04.005. Epub 2024 Apr 6. Mol Ther. 2024. PMID: 38584391 Free PMC article.
-
Chimeric antigen receptor (CAR) T-cell therapy for people with relapsed or refractory diffuse large B-cell lymphoma.Cochrane Database Syst Rev. 2021 Sep 13;9(9):CD013365. doi: 10.1002/14651858.CD013365.pub2. Cochrane Database Syst Rev. 2021. PMID: 34515338 Free PMC article.
References
-
- Lee D.W., Santomasso B.D., Locke F.L., Ghobadi A., Turtle C.J., Brudno J.N., Maus M.V., Park J.H., Mead E., Pavletic S., et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol. Blood Marrow Transplant. 2019;25:625–638. doi: 10.1016/j.bbmt.2018.12.758. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

