Periodic Boosters of COVID-19 Vaccines Do Not Affect the Safety and Efficacy of Immune Checkpoint Inhibitors for Advanced Non-Small Cell Lung Cancer: A Longitudinal Analysis of the Vax-On-Third Study
- PMID: 40563598
- PMCID: PMC12191419
- DOI: 10.3390/cancers17121948
Periodic Boosters of COVID-19 Vaccines Do Not Affect the Safety and Efficacy of Immune Checkpoint Inhibitors for Advanced Non-Small Cell Lung Cancer: A Longitudinal Analysis of the Vax-On-Third Study
Abstract
Background: Increasing evidence suggests that the immunogenicity of COVID-19 mRNA vaccines might influence the efficacy of immune checkpoint inhibitors (ICIs). Current studies have not considered the impact of additional vaccinations, which are now recommended as a preventive strategy against SARS-CoV-2 infection for cancer patients receiving active treatments. Consequently, we leveraged the prospective monitoring from the Vax-On-Third study to explore whether periodic mRNA vaccine boosters administered around the start of ICIs could influence the rates of immune-related adverse events (irAEs) and survival outcomes in patients with advanced non-small cell lung cancer (NSCLC).
Methods: Our study included patients with a histological diagnosis of metastatic NSCLC and available PD-L1 tumor proportion score (TPS), who had undergone at least two cycles of upfront treatment with pembrolizumab, cemiplimab, or their combination with platinum-based chemotherapy. Patients who received any additional mRNA-based vaccine doses within 60 days before to 30 days after starting ICIs accounted for the exposed cohort. Those who declined further boosters formed the reference cohort. We analyzed differences in irAE frequencies, progression-free survival (PFS), and overall survival (OS) using univariate and multivariate analyses.
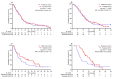
Results: Between 27 November 2021 and 31 March 2024, we enrolled 226 eligible patients. The exposed cohort consisted of 112 patients who had received either a third or fourth dose of tozinameran or a bivalent booster. Based on PD-L1 expression levels, 93 (41%) and 133 (59%) patients received single-agent ICIs (PD-L1 TPS ≥ 50%) or combination regimens (PD-L1 TPS < 50%), respectively. Propensity-score matching using comprehensive criteria resulted in two cohorts of 102 patients each, with an optimal balance of prognostic factors. A thorough analysis of any grade irAEs showed no significant differences between the cohorts. A longitudinal survival assessment with a median follow-up of 22.8 (95% CI 19.2-26.0) months showed no difference between the cohorts. The median PFS for the reference and exposed cohorts was 7.5 (95% CI 5.9-9.1) and 8.2 (95% CI 6.2-10.2) months, respectively (p = 0.408; HR 0.88 [95% CI 0.66-1.18]). The median OS for the reference and exposed cohorts was 10.5 (95% CI 7.9-13.0) and 13.8 (95% CI 12.0-15.5) months, respectively (p = 0.170; HR 0.81 [95% CI 0.59-1.09]). Multivariate analysis confirmed that receiving additional mRNA vaccine boosters did not significantly affect the risk of disease progression or mortality. Univariate analysis within the subgroup of patients with high PD-L1 TPS who received single-agent ICIs showed a significant OS advantage for patients in the exposed cohort (9.7 [95% CI 8.1-11.2] vs. 18.6 [95% CI 13.5-23.6] months; p = 0.034; HR 0.59 [95% CI 0.36-0.96]).
Conclusion: After optimally balancing prognostic factors, regular mRNA vaccine boosters at the onset of ICIs did not impact the safety and survival of patients with advanced NSCLC. The improved outcome observed in patients with high PD-L1 expression levels aligns with previous findings and warrants further investigation.
Keywords: COVID-19; booster dose; first-line therapy; immune checkpoint inhibitors; immune-related adverse events; mRNA vaccines; non-small-cell lung cancer; survival.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- World Health Organization WHO Coronavirus (COVID-19) Dashboard. [(accessed on 31 March 2025)]. Available online: https://data.who.int/dashboards/covid19/cases.
-
- McAuley H.J.C., Evans R.A., Bolton C.E., Brightling C.E., Chalmers J.D., Docherty A.B., Elneima O., Greenhaff P.L., Gupta A., Harris V.C., et al. Prevalence of physical frailty, including risk factors, up to 1 year after hospitalisation for COVID-19 in the UK: A multicentre, longitudinal cohort study. eClinicalMedicine. 2023;57:101896. doi: 10.1016/j.eclinm.2023.101896. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

