Future Perspectives in Senescence-Based Therapies for Head and Neck Cancer
- PMID: 40563615
- PMCID: PMC12190458
- DOI: 10.3390/cancers17121965
Future Perspectives in Senescence-Based Therapies for Head and Neck Cancer
Abstract
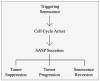
Cellular senescence is a complex physiological process in which cells permanently stop dividing and enter a stable state of cell-cycle arrest. This mechanism is typically triggered by various stressors, such as DNA damage, oxidative stress, telomere shortening, and oncogene activation. Senescent cells remain metabolically active and significantly influence their microenvironment through the senescence-associated secretory phenotype (SASP), which includes the secretion of inflammatory cytokines, growth factors, and proteases. While cellular senescence serves as a crucial tumor-suppressive mechanism by preventing the proliferation of damaged or potentially cancerous cells, it also plays a paradoxical role by promoting chronic inflammation, tissue dysfunction, and potentially oncogenesis. Therefore, understanding the regulation and impact of cellular senescence is vital for developing therapeutic interventions that leverage its benefits while minimizing adverse outcomes. In this review, we provide an overview of the current understanding of cellular senescence in cancer biology and discuss the emerging field of senescence-targeted therapies. We focus specifically on the role of senescence in head and neck cancers, examining the potential of induced senescence therapy to mitigate the progression of these tumors. This review aims to correlate the dual nature of senescence with innovative therapeutic strategies, highlighting its promise and challenges in improving treatment outcomes for HNC patients.
Keywords: HNSCC; biomarkers; cancer therapy; cellular senescence; chromatin organization; epigenetic regulation; therapy-induced senescence.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Multi-omics strategies to decode the molecular landscape of cellular senescence.Ageing Res Rev. 2025 Sep;111:102824. doi: 10.1016/j.arr.2025.102824. Epub 2025 Jul 5. Ageing Res Rev. 2025. PMID: 40618933 Review.
-
Psychological interventions for adults who have sexually offended or are at risk of offending.Cochrane Database Syst Rev. 2012 Dec 12;12(12):CD007507. doi: 10.1002/14651858.CD007507.pub2. Cochrane Database Syst Rev. 2012. PMID: 23235646 Free PMC article.
-
Harnessing the interaction between redox signaling and senescence to restrain tumor drug resistance.Front Cell Dev Biol. 2025 Jul 9;13:1639772. doi: 10.3389/fcell.2025.1639772. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40703657 Free PMC article. Review.
-
Senescence in cancer.Cancer Cell. 2025 Jul 14;43(7):1204-1226. doi: 10.1016/j.ccell.2025.05.015. Epub 2025 Jun 12. Cancer Cell. 2025. PMID: 40513577 Review.
References
Publication types
LinkOut - more resources
Full Text Sources

