Iterative Cytoreductive Surgery and HIPEC for Peritoneal Metastases from Primary Appendiceal and Colorectal Cancers: An Observational Study
- PMID: 40563664
- PMCID: PMC12191129
- DOI: 10.3390/cancers17122014
Iterative Cytoreductive Surgery and HIPEC for Peritoneal Metastases from Primary Appendiceal and Colorectal Cancers: An Observational Study
Abstract
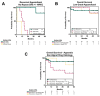
Background: Peritoneal relapse after cytoreduction and hyperthermic intraperitoneal chemotherapy (CRS/HIPEC) is common. Repeat CRS/HIPEC offers the potential for long-term survival in the appropriately selected patient. Methods: We performed a retrospective review of a single institution database to assess perioperative outcomes after repeat CRS/HIPEC for appendiceal (pAC) and colorectal (pCRC) cancers. Kaplan-Meier and Cox estimates were used to assess survival. Results: Of 157 patients, 103 patients underwent initial CRS/HIPEC for pAC (n = 67) or pCRC (n = 36) histologies. Twenty-seven pAC patients (27/67, 40%) and 23/36 pCRC patients (63%) developed disease recurrence. Relapsed patients had a higher burden of disease (PCI), operative length and blood loss and received adjuvant chemotherapy (all p < 0.05). Nine of the 27 relapsed pAC patients and 5 of the 13 relapsed pCRC patients underwent repeat CRS/HIPEC. The median time to repeat CRS/HIPEC was 18 months (4-26 months), and a CCR-0 and CCR-1 were achieved in 79% and 21%, respectively. The 1-, 3- and 5-year OS for pAC patients who underwent repeat CRS/HIPEC was 88.9%, 88.9% and 77.8%, and the 1- and 3-year OS for pCRC patients was 100% and 25%, respectively. Repeat CRS/HIPEC for pAC was associated with significant improvement in OS (p = 0.03), while for pCRC, no significant difference was observed (p = 0.99). Conclusions: Repeat CRS/HIPEC for isolated peritoneal recurrence is safe and offers the potential for long-term survival. Patient selection is key to ensure optimal cytoreduction when considering repeat CRS/HIPEC.
Keywords: CRS/HIPEC; HIPEC; cytoreduction; peritoneal carcinomatosis; recurrence; repeat.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Chua T.C., Moran B.J., Sugarbaker P.H., Levine E.A., Glehen O., Gilly F.N., Baratti D., Deraco M., Elias D., Sardi A., et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J. Clin. Oncol. 2012;30:2449–2456. doi: 10.1200/JCO.2011.39.7166. - DOI - PubMed
-
- Verwaal V.J., Bruin S., Boot H., van Slooten G., van Tinteren H. 8-year follow-up of randomized trial: Cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann. Surg. Oncol. 2008;15:2426–2432. doi: 10.1245/s10434-008-9966-2. - DOI - PubMed
-
- Verwaal V.J., Boot H., Aleman B.M.P., van Tinteren H., Zoetmulder F.A.N. Recurrences after peritoneal carcinomatosis of colorectal origin treated by cytoreduction and hyperthermic intraperitoneal chemotherapy: Location, treatment, and outcome. Ann. Surg. Oncol. 2004;11:375–379. doi: 10.1245/ASO.2004.08.014. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

