Redefining the Use of Regorafenib and Trifluridine/Tipiracil Without Bevacizumab in Refractory Metastatic Colorectal Cancer: Findings from the ReTrITA Study
- PMID: 40563686
- PMCID: PMC12191189
- DOI: 10.3390/cancers17122037
Redefining the Use of Regorafenib and Trifluridine/Tipiracil Without Bevacizumab in Refractory Metastatic Colorectal Cancer: Findings from the ReTrITA Study
Abstract
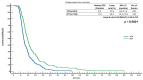
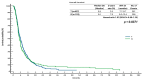
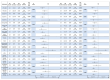
Background: Regorafenib (R) and trifluridine/tipiracil (T) are approved treatments for metastatic colorectal cancer (mCRC) in refractory cases. However, the optimal sequencing of these agents is unknown. The ReTrITA study planned to assess the real-world efficacy of R and T, administered either sequentially or as monotherapy, in a large Italian multicentre population. Methods: This retrospective observational analysis comprised 1156 mCRC patients treated between 2012 and 2023 at 17 Italian cancer centres. Patients were divided into four groups: sequential T/R (n = 261), sequential R/T (n = 155), R monotherapy (n = 313), and T monotherapy (n = 427). The primary objectives were overall survival (OS) and progression-free survival (PFS), with secondary goals being disease control rate, objective response rate, and treatment-related toxicity. Results: The monotherapy cohorts showed no significant difference in OS (R: 5.0 months; T: 5.9 months; p = 0.8371) or PFS (R: 3.2 months; T: 3.3 months; p = 0.6531). Compared to T/R, the sequential R/T group had significantly better outcomes: median OS was 16.6 vs. 12.6 months (HR = 0.67; p = 0.0004), and median PFS was 11.5 vs. 8.5 months (HR = 0.60; p < 0.0001). The survival advantage of R/T was consistent across clinical subgroups. The toxicity profiles were comparable with known safety data, with a lower prevalence of neutropenia reported in the R/T sequence. Conclusions: ReTrITA confirms the efficacy of R and T as monotherapies and provides compelling real-world evidence that the R/T sequence improves survival in refractory mCRC. These findings support a regorafenib-first approach in patients who are eligible, and they emphasise the need for future research into combination strategies and comparisons with newer drugs such as fruquintinib.
Keywords: metastatic colorectal cancer; real-world evidence; regorafenib; sequencing; survival; toxicity; trifluridine/tipiracil.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Colon Cancer Version 3.2025—24 April 2025. [(accessed on 10 May 2025)]. Available online: www.nccn.org/professionals/physician_gls/pdf/colon.pdf.
-
- National Cancer Institute Cancer Stat Facts: Colorectal Cancer. [(accessed on 10 May 2025)]; Available online: https://seer.cancer.gov/statfacts/html/colorect.html.
-
- Van Cutsem E., Nordlinger B., Adam R., Köhne C.H., Pozzo C., Poston G., Ychou M., Rougier P., European Colorectal Metastases Treatment Group Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. Eur. J. Cancer. 2006;42:2212–2221. doi: 10.1016/j.ejca.2006.04.012. - DOI - PubMed
-
- Kemeny N. Management of liver metastases from colorectal cancer. Oncology. 2006;20:1161–1176, 1179. discussion 1179–1180, 1185–1186. - PubMed
LinkOut - more resources
Full Text Sources

