Movement Impairments May Not Preclude Visuomotor Adaptation After Stroke
- PMID: 40563790
- PMCID: PMC12191063
- DOI: 10.3390/brainsci15060619
Movement Impairments May Not Preclude Visuomotor Adaptation After Stroke
Abstract
Purpose: Many individuals with stroke partake in rehabilitation to improve their movements. Rehabilitation operates on the assumption that individuals with stroke can use visual feedback from their movements or visual cues from a therapist to improve their movements through practice. However, this type of visuomotor learning can be impaired after stroke. It is unclear whether and how learning impairments relate to impairments in movement. Here, we examined the relationship between learning and movement impairments after stroke.
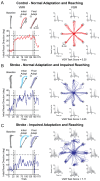
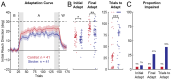
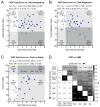
Methods: We recruited adults with first-time unilateral stroke and controls matched for overall age and sex. The participants performed a visuomotor learning task in a Kinarm exoskeleton robot. The task assessed how they adapted their reaching movements to a systematic visual disturbance that altered the relationship between the observed and actual motion of their hand. Learning was quantified as the extent to which the participants adapted their movements to the visual disturbance. A separate visually-guided reaching task was used to assess the straightness, direction, smoothness, and duration of their movements. The relationships between visuomotor adaptation and movement were analyzed using Spearman's correlations. Control data were used to identify impairments in visuomotor adaptation and movement. The independence of these impairments was examined using Fisher's exact tests.
Results: Impairments in visuomotor adaptation (46.3%) and movement (73.2%) were common in participants with stroke (n = 41). We observed weak-moderate correlations between continuous measures of adaptation and movement performance (rho range: -0.44-0.58). Adaptation and movement impairments, identified using the range of performance in the control participants, were statistically independent (all p > 0.05).
Conclusions: Movement impairments accounted for 34% of the variance in visuomotor adaptation at best. Our findings suggest that factors other than movement impairments may influence visuomotor adaptation after stroke.
Keywords: motor adaptation; motor learning; movement impairment; robotics; stroke recovery; stroke rehabilitation; upper limb.
Conflict of interest statement
The author(s) declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures


Similar articles
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Bioengineered nerve conduits and wraps for peripheral nerve repair of the upper limb.Cochrane Database Syst Rev. 2022 Dec 7;12(12):CD012574. doi: 10.1002/14651858.CD012574.pub2. Cochrane Database Syst Rev. 2022. PMID: 36477774 Free PMC article.
-
The educational effects of portfolios on undergraduate student learning: a Best Evidence Medical Education (BEME) systematic review. BEME Guide No. 11.Med Teach. 2009 Apr;31(4):282-98. doi: 10.1080/01421590902889897. Med Teach. 2009. PMID: 19404891
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Intravenous magnesium sulphate and sotalol for prevention of atrial fibrillation after coronary artery bypass surgery: a systematic review and economic evaluation.Health Technol Assess. 2008 Jun;12(28):iii-iv, ix-95. doi: 10.3310/hta12280. Health Technol Assess. 2008. PMID: 18547499
References
-
- Krakauer J.W. Motor Learning: Its Relevance to Stroke Recovery and Neurorehabilitation. Curr. Opin. Neurol. 2006;19:84–90. doi: 10.1097/01.wco.0000200544.29915.cc. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

