DoDELLA-GAI2 Integrates Gibberellin and Ethylene Signaling to Regulate Chinese Yam (Dioscorea opposita) Tuber Development
- PMID: 40563886
- PMCID: PMC12189092
- DOI: 10.3390/biology14060635
DoDELLA-GAI2 Integrates Gibberellin and Ethylene Signaling to Regulate Chinese Yam (Dioscorea opposita) Tuber Development
Abstract
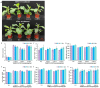
Yam (Dioscorea opposita) tuber development is a complex process regulated by various phytohormones, with gibberellin (GA) playing a crucial role. However, the underlying mechanisms and interaction of GA with other phytohormone pathways on yam tuber development remain incompletely understood. This study investigated the regulatory role of GA and its crosstalk with other phytohormones during yam tuber growth through phenotypic, cytological, physiological, and transcriptomic as well as targeted phytohormone metabolomics analyses. The results reveal that exogenous GA promoted tuber enlargement increases vascular bundle and the number and diameter of sieve tubes, and alters the expression of GA anabolism genes and GA signal transduction pathways. Integrated transcriptome and targeted metabolomics analyses revealed coordinated changes in GA and ethylene (ETH) biosynthesis and signaling pathways during tuber development, particularly DELLA-GAI2 acting as a negative regulator of GA signaling. Overexpression of DoDELLA-GAI2 in transgenic tobacco significantly reduced GA level, starch, cytokinin (CTK), and ETH content, as well as aerenchyma tissue growth and parenchyma cell size. Exogenous GA and ethephon treatments increased GA, starch, CTK, and ETH content, and downregulated DoDELLA-GAI2 gene expression. The yeast two-hybrid and bimolecular fluorescence complementation (BiFC) assays confirmed a direct interaction between DoDELLA-GAI2 and DoMTCPB, an upstream gene-encoding key enzyme in ETH biosynthesis. DoDELLA-GAI2 acts as a negative regulator of ETH synthesis by interacting with DoMTCPB. GA-induced degradation of DoDELLA-GAI2 relieves this inhibition, promoting ETH production and contributing to tuber growth. Taken together, our findings reveal a novel mechanism based on DoDELLA-GAI2 integrating the GA and ETH signaling processes to regulate tuber development in D. opposita, offering a potential target for improving yam crop productivity.
Keywords: DELLA-GAI; Dioscorea opposita; gibberellin; interaction mechanism; signaling cascade reaction.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures














Similar articles
-
Genome-Wide Characterization of DrRALF Genes in Yam (Dioscorea rotundata) Reveals Their Potential Roles in Tuber Expansion and the Gibberellin Response.Int J Mol Sci. 2025 Jun 26;26(13):6151. doi: 10.3390/ijms26136151. Int J Mol Sci. 2025. PMID: 40649928 Free PMC article.
-
A Non-Specific Phytohormone Regulatory Network in Saccharina japonica Coordinates Growth and Environmental Adaptation.Plants (Basel). 2025 Jun 13;14(12):1821. doi: 10.3390/plants14121821. Plants (Basel). 2025. PMID: 40573810 Free PMC article.
-
Metabolome and transcriptome profiling reveals light-induced anthocyanin biosynthesis and anthocyanin-related key transcription factors in Yam (Dioscorea Alata L.).BMC Plant Biol. 2025 May 30;25(1):729. doi: 10.1186/s12870-025-06738-w. BMC Plant Biol. 2025. PMID: 40442608 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: a systematic review and economic evaluation.Health Technol Assess. 2006 Aug;10(28):iii-iv, xi-xiv, 1-183. doi: 10.3310/hta10280. Health Technol Assess. 2006. PMID: 16904047
References
-
- Li Y., Ji S., Xu T., Zhong Y., Xu M., Liu Y., Li M., Fan B., Wang F., Xiao J., et al. Chinese yam (Dioscorea): Nutritional value, beneficial effects, and food and pharmaceutical applications. Trends Food Sci. Technol. 2023;134:29–40. doi: 10.1016/j.tifs.2023.01.021. - DOI
-
- Datir S., Kumbhar R., Kumatkar P. Understanding physiological and biochemical mechanisms associated with post-harvest storage of yam tuber (Dioscorea sp.) Technol. Hortic. 2024;4:e004. doi: 10.48130/tihort-0024-0001. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

