Maternal Overnutrition in Beef Cattle: Effects on Fetal Programming, Metabolic Health, and Postnatal Outcomes
- PMID: 40563896
- PMCID: PMC12190084
- DOI: 10.3390/biology14060645
Maternal Overnutrition in Beef Cattle: Effects on Fetal Programming, Metabolic Health, and Postnatal Outcomes
Abstract
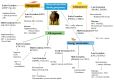
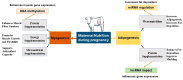
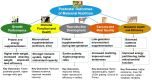
Maternal overnutrition and targeted supplements during pregnancy strongly affect fetal development in beef cattle, influencing gene expression, tissue development, and productivity after birth. As modern feeding practices often result in cows receiving energy and protein above requirements, understanding the balance between adequate nutrition and overconditioning is critical for sustainable beef production. This review synthesizes findings from recent studies on maternal overnutrition and supplementation, focusing on macronutrients (energy, protein, methionine) and key micronutrients (e.g., selenium, zinc). It evaluates the timing and impact of supplementation during different gestational stages, with emphasis on fetal muscle and adipose tissue development, immune function, and metabolic programming. The role of epigenetic mechanisms, such as DNA methylation and non-coding RNAs, is also discussed in relation to maternal dietary inputs. Mid-gestation supplementation promotes muscle growth by activating muscle-specific genes, whereas late-gestation diets enhance marbling and carcass traits. However, maternal overnutrition may impair mitochondrial efficiency, encourage fat deposition over muscle, and promote collagen synthesis, reducing meat tenderness. Recent evidence highlights sex-specific fetal programming differences, the significant impact of maternal diets on offspring gut microbiomes, and breed-specific nutritional responses, and multi-OMICs integration reveals metabolic reprogramming mechanisms. Targeted trace mineral and methionine supplementation enhance antioxidant capacity, immune function, and reproductive performance. Precision feeding strategies aligned with gestational requirements improve feed efficiency and minimize overfeeding risks. Early interventions, including protein and vitamin supplementation, optimize placental function and fetal development, supporting stronger postnatal growth, immunity, and fertility. Balancing nutritional adequacy without excessive feeding supports animal welfare, profitability, and sustainability in beef cattle systems.
Keywords: adipogenesis; beef cattle production; fetal programming; maternal nutrition; muscle development.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Bell A.W., Greenwood P.L. Prenatal origins of postnatal variation in growth, development and productivity of ruminants. Anim. Prod. Sci. 2016;56:1217–1232. doi: 10.1071/AN15408. - DOI
-
- Govoni K.E., Reed S.A., Zinn S.A. Cell biology symposium: Metabolic responses to stress: From animal to cell: Poor maternal nutrition during gestation: Effects on offspring whole-body and tissue-specific metabolism in livestock species. J. Anim. Sci. 2019;97:3142–3152. doi: 10.1093/jas/skz157. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

