DNA Methylation of Igf2r Promoter CpG Island 2 Governs Cis-Acting Inheritance and Gene Dosage in Equine Hybrids
- PMID: 40563929
- PMCID: PMC12189881
- DOI: 10.3390/biology14060678
DNA Methylation of Igf2r Promoter CpG Island 2 Governs Cis-Acting Inheritance and Gene Dosage in Equine Hybrids
Abstract
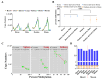
Genomic imprinting is critical for mammalian development, but its regulation varies across species. The insulin-like growth factor 2 receptor (IGF2R), which is a maternally expressed imprinted gene critical for cell proliferation and differentiation, as well as embryonic and placental development, is classically regulated by differentially methylated regions (DMRs) and lncRNA-Airn in mice. However, studies on this in equus are scarce, especially in terms of mechanistic studies. In the present study, heart, liver, spleen, lung, kidney, brain, and muscle samples were obtained from horses, donkeys, and hybrids, and gene expression and imprinting state were tested to investigate the imprinting regulation of Igf2r in these animals. Bisulfite sequencing combined with an allele-specific expression analysis revealed a tissue-specific loss of imprinting in the mule liver and hybrid brain tissues. Strikingly, we found that the maternal-specific expression of equine Igf2r did not rely on the canonical DMRs or lncRNA-Airn. Surprisingly, DNA methylation of a specific region called CpG island 2 (CpGI2) in the Igf2r promoter showed cis-acting inheritance, meaning that the DNA methylation patterns of the parental alleles are retained in hybrid tissues. Notably, the DNA methylation of CpGI2 correlated negatively with Igf2r expression in the spleen (R2 = 0.8797, p = 6.46 × 10-6), lung (R2 = 0.8569, p = 1.57 × 10-5), and kidney (R2 = 0.8650, p = 3.85 × 10-6). Our findings suggest that imprinting may work differently in other species. This study provides a framework for understanding imprinting diversity in hybrids and shows that equine hybrids can be used to study how epigenetic inheritance works.
Keywords: DNA methylation; Igf2r; cis-acting inheritance; epigenetic regulation; equus; genomic imprinting.
Conflict of interest statement
Yingchao Shen was employed by the company Anchee (Shandong) Animal Nutrition Research Academy Co., Ltd., Jinan, Shandong. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

