Adjunct Therapy with Ipragliflozin Exerts Limited Effects on Kidney Protection in Type 1 Diabetes: A Retrospective Study Conducted at 25 Centers in Japan (IPRA-CKD)
- PMID: 40564007
- PMCID: PMC12189841
- DOI: 10.3390/biomedicines13061287
Adjunct Therapy with Ipragliflozin Exerts Limited Effects on Kidney Protection in Type 1 Diabetes: A Retrospective Study Conducted at 25 Centers in Japan (IPRA-CKD)
Abstract
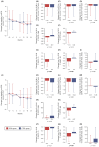
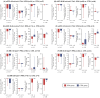
Background/Objectives: While sodium-glucose cotransporter 2 (SGLT2) inhibitors have demonstrated additional non-glycemic benefits for renal protection in individuals with type 2 diabetes, less evidence is available for those with type 1 diabetes (T1D). To determine whether the adjunctive use of the SGLT2 inhibitor ipragliflozin confers kidney protection in individuals with T1D, we retrospectively analyzed data from a real-world cohort examined at 25 centers in Japan. Methods: We enrolled 359 subjects aged 20-74 years with T1D (IPRA group: 159 ipragliflozin users; control [CTRL] group: 200 non-users). The primary outcome was changes in the estimated glomerular filtration rate (eGFR) from baseline to 24 months after the initiation of ipragliflozin. The secondary outcomes were all other changes, including the urinary albumin-creatinine ratio (UACR) and urinary protein-creatinine ratio (UPCR). Results: The IPRA group's eGFR decline slopes were 0.79 mL/min/1.73 m2/year milder than the CTRL group's after propensity score matching, but this difference was not significant. The subjects complicated by chronic kidney disease (CKD) defined as UACR ≥ 30 mg/g and/or UPCR ≥ 0.5 g/g and/or eGFR < 60 mL/min/1.73 m2 showed changes in UPCR (g/g) from baseline to 24 months that were significantly lower in the IPRA group (-0.27 ± 1.63) versus the CTRL group (0.18 ± 0.36) (p = 0.016). No significant increase in adverse events (including severe hypoglycemia and hospitalization due to ketosis/ketoacidosis or cardiovascular diseases) was observed in the IPRA group. Conclusions: Adjunctive treatment with ipragliflozin exerted potential renal benefits by decreasing proteinuria in T1D subjects with CKD. Further investigations are required to determine whether its additional benefits exceed the increased risk of ketoacidosis.
Keywords: SGLT2 inhibitor; chronic kidney disease; ipragliflozin; renal outcome; type 1 diabetes.
Conflict of interest statement
The funder had no role in the design of this study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
-
- Waijer S.W., Vart P., Cherney D.Z.I., Chertow G.M., Jongs N., Langkilde A.M., Mann J.F.E., Mosenzon O., McMurray J.J.V., Rossing P., et al. Effect of dapagliflozin on kidney and cardiovascular outcomes by baseline KDIGO risk categories: A post hoc analysis of the DAPA-CKD trial. Diabetologia. 2022;65:1085–1097. doi: 10.1007/s00125-022-05694-6. - DOI - PMC - PubMed
-
- Jhund P.S., Kondo T., Butt J.H., Docherty K.F., Claggett B.L., Desai A.S., Vaduganathan M., Gasparyan S.B., Bengtsson O., Lindholm D., et al. Dapagliflozin across the range of ejection fraction in patients with heart failure: A patient-level, pooled meta-analysis of DAPA-HF and DELIVER. Nat. Med. 2022;28:1956–1964. doi: 10.1038/s41591-022-01971-4. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

