Ventricular Arrhythmias and Myocardial Infarction: Electrophysiological and Neuroimmune Mechanisms
- PMID: 40564008
- PMCID: PMC12189811
- DOI: 10.3390/biomedicines13061290
Ventricular Arrhythmias and Myocardial Infarction: Electrophysiological and Neuroimmune Mechanisms
Abstract
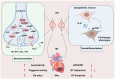
Ventricular arrhythmias (VAs) after myocardial infarction (MI) are still one of the most important causes of cardiovascular death, though patients receive timely vascular recanalization and drug treatment. And it requires further exploring the mechanism and new therapeutics of VAs induced by MI. Here, we review the electrophysiological and neuroimmune mechanisms of VAs induced by MI. Immune cells are regulated by combining with neuroendocrine molecules released by the sympathetic nervous system (SNS), and, in turn, they modulate SNS both at the paraventricular nucleus of the hypothalamus and stellate ganglion by releasing cytokines or chemokines. In addition, 'life essentials' such as sleep, physiological health, and exercise can also influence cardiovascular health through neuroimmune mechanisms. Those factors and mechanisms provide us with new perspectives for understanding the occurrence and maintenance of VAs after MI. Exploring the crosstalk between electrophysiology and neuroimmunology will contribute to finding new therapeutics for VAs after MI.
Keywords: crosstalk; immune system; myocardial infarction; sympathetic nervous system; ventricular arrhythmias.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
-
- Roth G.A., Mensah G.A., Johnson C.O., Addolorato G., Ammirati E., Baddour L.M., Barengo N.C., Beaton A.Z., Benjamin E.J., Benziger C.P., et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010. Erratum in J. Am. Coll. Cardiol. 2021, 77, 1958–1959. - DOI - PMC - PubMed
-
- Garcia R., Marijon E., Karam N., Narayanan K., Anselme F., Cesari O., Champ-Rigot L., Manenti V., Martins R., Puymirat E., et al. Ventricular fibrillation in acute myocardial infarction: 20-year trends in the FAST-MI study. Eur. Heart J. 2022;43:4887–4896. doi: 10.1093/eurheartj/ehac579. - DOI - PubMed
-
- Vallabhajosyula S., Patlolla S.H., Verghese D., Ya’Qoub L., Kumar V., Subramaniam A.V., Cheungpasitporn W., Sundaragiri P.R., Noseworthy P.A., Mulpuru S.K., et al. Burden of Arrhythmias in Acute Myocardial Infarction Complicated by Cardiogenic Shock. Am. J. Cardiol. 2020;125:1774–1781. doi: 10.1016/j.amjcard.2020.03.015. - DOI - PMC - PubMed
-
- Kosmidou I., Embacher M., McAndrew T., Dizon J.M., Mehran R., Ben-Yehuda O., Mintz G.S., Stone G.W. Early Ventricular Tachycardia or Fibrillation in Patients with ST Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention and Impact on Mortality and Stent Thrombosis (from the Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction Trial) Am. J. Cardiol. 2017;120:1755–1760. doi: 10.1016/j.amjcard.2017.07.080. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

