Prognostic Significance of Delays in Initiation of Adjuvant Trastuzumab-Based Therapy in Patients with HER2-Positive Breast Cancer
- PMID: 40564024
- PMCID: PMC12189480
- DOI: 10.3390/biomedicines13061305
Prognostic Significance of Delays in Initiation of Adjuvant Trastuzumab-Based Therapy in Patients with HER2-Positive Breast Cancer
Abstract
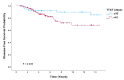
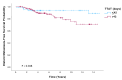
Purpose: Adjuvant trastuzumab therapy has improved outcomes in HER2-positive breast cancer, but the impact of the timing of its initiation remains unclear. This study evaluates the effect of time to adjuvant trastuzumab-based therapy (TTAT) after surgery on survival in HER2-positive breast cancer. Methods: In this retrospective study, HER2-positive breast cancer patients treated with surgery followed by adjuvant trastuzumab without prior neoadjuvant therapy were analyzed. Patients were grouped by TTAT ≤ 42 days or >42 days post-surgery. Key endpoints included overall survival (OS), disease-free survival (DFS), and distant metastasis-free survival (DMFS), evaluated through Kaplan-Meier and Cox regression analyses. Results: Patients with TTAT greater than 42 days had significantly worse OS, DFS, and DMFS (p = 0.036, p = 0.045, and p = 0.048, respectively, log-rank test) than those initiating trastuzumab within 42 days. On multivariate analysis, delays beyond 42 days were associated with a significantly increased risk of recurrence and mortality, showing reduced DFS (HR 2.52; p = 0.027) and OS (HR 4.48; p = 0.004). These findings indicate that even moderate delays in trastuzumab initiation can adversely affect survival. Conclusions: Delaying trastuzumab initiation beyond 42 days post-surgery negatively impacts survival in HER2-positive breast cancer, emphasizing the need for timely treatment. These results support clinical guidelines advocating prompt initiation of adjuvant therapy to improve long-term outcomes for HER2-positive patients.
Keywords: adjuvant therapy; breast cancer; breast surgery; targeted therapy; trastuzumab.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Joensuu H., Bono P., Kataja V., Alanko T., Kokko R., Asola R., Utriainen T., Turpeenniemi-Hujanen T., Jyrkkiö S., Möykkynen K., et al. Fluorouracil, epirubicin, and cyclophosphamide with either docetaxel or vinorelbine, with or without trastuzumab, as adjuvant treatments of breast cancer: Final results of the FinHer Trial. J. Clin. Oncol. 2009;27:5685–5692. doi: 10.1200/JCO.2008.21.4577. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

