Role of Cellular Senescence in Parkinson's Disease: Potential for Disease-Modification Through Senotherapy
- PMID: 40564120
- PMCID: PMC12190713
- DOI: 10.3390/biomedicines13061400
Role of Cellular Senescence in Parkinson's Disease: Potential for Disease-Modification Through Senotherapy
Abstract
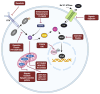
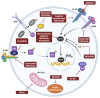
Parkinson's disease (PD) is an aging-related neurodegenerative disease characterized by a progressive loss of dopamine (DA)-secreting neurons in the substantia nigra. Most of the currently available treatments attempt to alleviate the disease symptoms by increasing DA transmission in the brain and are associated with unpleasant side effects. Since there are no treatments that modify the course of PD or regenerate DA neurons, identifying therapeutic strategies that slow, stop, or reverse cell death in PD is of critical importance. Here, factors that confer vulnerability of substantia nigra DA neurons to cell death and the primary mechanisms of PD pathogenesis, including cellular senescence, a cellular stress response that elicits a stable cell cycle arrest in mitotic cells and profound phenotypic changes including the implementation of a pro-inflammatory secretome, are reviewed. Additionally, a discussion of the characteristics, mechanisms, and markers of cellular senescence and the development of approaches to target senescent cells, referred to as senotherapeutics, is included. Although the senotherapeutics curcumin, fisetin, GSK-650394, and astragaloside IV had disease-modifying effects in in vitro and in vivo models of PD, the potential long-term side effects of these compounds remain unclear. It remains to be elucidated whether their beneficial effects will translate to non-human primate models and/or human PD patients. The enhanced selectivity, safety, and/or efficacy of next generation senotherapeutic strategies including senolytic peptides, senoreverters, proteolysis-targeting chimeras, pro-drugs, immunotherapy, and nanoparticles will also be reviewed. Although these next generation senotherapeutics may have advantages, none have been tried in models of PD.
Keywords: Parkinson’s disease; cellular senescence; neurodegeneration; pathology; therapeutics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Use of β-adrenoreceptor drugs and Parkinson's disease incidence in women from the French E3N cohort study.J Parkinsons Dis. 2025 Jun;15(4):789-804. doi: 10.1177/1877718X251330993. Epub 2025 Apr 29. J Parkinsons Dis. 2025. PMID: 40302366
-
Antidepressants for pain management in adults with chronic pain: a network meta-analysis.Health Technol Assess. 2024 Oct;28(62):1-155. doi: 10.3310/MKRT2948. Health Technol Assess. 2024. PMID: 39367772 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Behavioral interventions to reduce risk for sexual transmission of HIV among men who have sex with men.Cochrane Database Syst Rev. 2008 Jul 16;(3):CD001230. doi: 10.1002/14651858.CD001230.pub2. Cochrane Database Syst Rev. 2008. PMID: 18646068
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
References
Publication types
LinkOut - more resources
Full Text Sources

