Pharmacological and Pathological Implications of Sigma-1 Receptor in Neurodegenerative Diseases
- PMID: 40564128
- PMCID: PMC12190457
- DOI: 10.3390/biomedicines13061409
Pharmacological and Pathological Implications of Sigma-1 Receptor in Neurodegenerative Diseases
Abstract
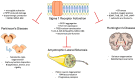
Originally identified as a potential receptor for opioids, the sigma-1 receptor is now recognized as an intracellular chaperone protein associated with mitochondria-associated membranes at the endoplasmic reticulum (ER). Over the past two decades, extensive research has revealed that the sigma-1 receptor regulates many cellular processes, such as calcium homeostasis, oxidative stress responses, protein folding, and mitochondrial function. The various functions of the sigma-1 receptor highlight its role as a central modulator of neuronal health and may be a promising pharmacological target across multiple neurodegenerative conditions. Herein, we provide an overview of the current pharmacological understanding of the sigma-1 receptor with an emphasis on the signaling mechanisms involved. We examine its pathological implications in common neurodegenerative diseases such as Alzheimer's disease, Parkinson's disease, amyotrophic lateral sclerosis, Huntington's disease, and multiple sclerosis. We then highlight how sigma-1 receptor modulation may influence disease progression as well as potential pharmacological mechanisms to alter disease outcomes. The translational potential of sigma-1 receptor therapies is discussed, as well as the most up-to-date results of ongoing clinical trials. This review aims to clarify the therapeutic potential of the sigma-1 receptor in neurodegeneration and guide future research in these diseases.
Keywords: Alzheimer’s disease; ER stress; TMEM97; amyotrophic lateral sclerosis; neurodegenerative disorders; sigma receptor; sigma-1 receptor.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Treatment for sialorrhea (excessive saliva) in people with motor neuron disease/amyotrophic lateral sclerosis.Cochrane Database Syst Rev. 2022 May 20;5(5):CD006981. doi: 10.1002/14651858.CD006981.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593746 Free PMC article.
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
Interventions for fatigue and weight loss in adults with advanced progressive illness.Cochrane Database Syst Rev. 2012 Jan 18;1:CD008427. doi: 10.1002/14651858.CD008427.pub2. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2017 Apr 07;4:CD008427. doi: 10.1002/14651858.CD008427.pub3. PMID: 22258985 Updated.
-
Interventions for palliative symptom control in COVID-19 patients.Cochrane Database Syst Rev. 2021 Aug 23;8(8):CD015061. doi: 10.1002/14651858.CD015061. Cochrane Database Syst Rev. 2021. PMID: 34425019 Free PMC article.
-
WITHDRAWN: Interventions for fatigue and weight loss in adults with advanced progressive illness.Cochrane Database Syst Rev. 2017 Apr 7;4(4):CD008427. doi: 10.1002/14651858.CD008427.pub3. Cochrane Database Syst Rev. 2017. PMID: 28387447 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

