Pharmacokinetics and Ex Vivo Activity of 7-Methylxanthine, an Inhibitor of Monosodium Urate Crystallization
- PMID: 40564129
- PMCID: PMC12190732
- DOI: 10.3390/biomedicines13061411
Pharmacokinetics and Ex Vivo Activity of 7-Methylxanthine, an Inhibitor of Monosodium Urate Crystallization
Abstract
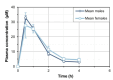
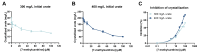
Background/Objectives: 7-Methylxanthine (7-MX) is a naturally occurring metabolite of caffeine and theobromine that can inhibit the crystallization of monosodium urate (MSU) and may be useful for the prevention or treatment of gout. However, the pharmacokinetics and ex vivo activity of 7-MX remain poorly characterized. Methods: The present study assessed the pharmacokinetics of 7-MX in Sprague Dawley rats following a single oral dose (30 mg/kg), and the ex vivo inhibition of MSU crystallization by 7-MX in rat plasma after the repeated administration of oral 7-MX. Results: The pharmacokinetic analysis showed that 7-MX reached peak plasma concentration (Cmax ≈ 30 µM) at 30 min after administration (tmax), the terminal half-life was approximately 1.4 h, and there was no evidence of accumulation after repeated daily dosing. After repeated administration, the relationship between dose (30 or 60 mg/kg) and plasma concentration was proportional. In vitro and ex vivo crystallization assays demonstrated that 7-MX inhibited MSU crystallization in a concentration-dependent manner. The in vitro studies showed that 100 µM 7-MX inhibited up to 74% of MSU crystallization under supersaturated conditions (400 mg/L urate). The ex vivo experiments indicated that plasma from rats that received 30 or 60 mg/kg of 7-MX had 41.4% and 52.6% inhibition of crystallization, consistent with the measured plasma concentrations. Conclusions: These findings confirm that oral administration of 7-MX to rats led to a plasma level that was sufficient to decrease MSU crystallization in plasma, and there were no observable toxicities. These results support the potential of 7-MX as a safe oral treatment for gout, especially in combination with urate-lowering therapies, such as allopurinol. Further clinical investigations are warranted to confirm the therapeutic potential of 7-MX in humans.
Keywords: 7-methylxanthine; gout; monosodium urate; oral administration; pharmacokinetics.
Conflict of interest statement
Author Joan Albertí was employed by the company ADMETRA Consulting. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
-
- Ashihara H., Kato M., Crozier A. Methylxanthines. Volume 200. Springer; Berlin/Heidelberg, Germany: 2011. Distribution, Biosynthesis and Catabolism of Methylxanthines in Plants; pp. 11–31. Handbook of Experimental Pharmacology. - PubMed
-
- Martínez-López S., Sarriá B., Gómez-Juaristi M., Goya L., Mateos R., Bravo-Clemente L. Theobromine, Caffeine, and Theophylline Metabolites in Human Plasma and Urine after Consumption of Soluble Cocoa Products with Different Methylxanthine Contents. Food Res. Int. 2014;63:446–455. doi: 10.1016/j.foodres.2014.03.009. - DOI
-
- Arnaud M.J. Methylxanthines. Volume 200. Springer; Berlin/Heidelberg, Germany: 2011. Pharmacokinetics and Metabolism of Natural Methylxanthines in Animal and Man; pp. 33–91. Handbook of Experimental Pharmacology. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

