Animal Models of Spinal Cord Injury
- PMID: 40564146
- PMCID: PMC12191375
- DOI: 10.3390/biomedicines13061427
Animal Models of Spinal Cord Injury
Abstract
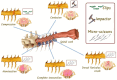
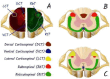
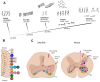
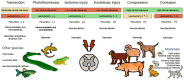
Spinal cord injury (SCI) is one of the most frequent causes of disability, accompanied by motor and postural impairments, as well as autonomic and behavioural disorders. Since the beginning of the last century, researchers have been developing and refining experimental models of SCI to study pathogenesis and find therapies. Since the beginning of the 20th century, quite a wide range of methods have been developed for contusion and compression injury, complete and partial transection of the spinal cord, and many others. The choice of model subject in such studies was not limited to mammals, but also included amphibians, lampreys, and even fish. Many functional tests have been proposed to assess functional recovery after injury in laboratory animals, ranging from simple rating scales to locomotion kinematics or recording of spinal neuronal activity. This review describes existing models of SCI in most animal species used in neurobiology. Their key characteristics are discussed, which determine the choice of model and model animals depending on the experimental tasks. Each experimental model of SCI has its own advantages and disadvantages determined by species-specific features of spinal cord anatomy and physiology, the speed of recovery from injury, and the ratio of the necrosis zone to the penumbra. The applicability and availability of the proposed methods for assessing the speed and completeness of recovery is also an important factor.
Keywords: animal models; cats; compression; contusion; dogs; fish; lampreys; mice; monkeys; pigs; rats; sheep; spinal cord injury; transection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



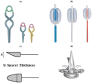
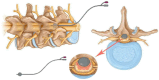
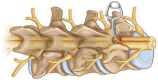
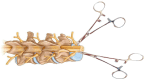


Similar articles
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
The clinical effectiveness and cost-effectiveness of enzyme replacement therapy for Gaucher's disease: a systematic review.Health Technol Assess. 2006 Jul;10(24):iii-iv, ix-136. doi: 10.3310/hta10240. Health Technol Assess. 2006. PMID: 16796930
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Assessing the comparative effects of interventions in COPD: a tutorial on network meta-analysis for clinicians.Respir Res. 2024 Dec 21;25(1):438. doi: 10.1186/s12931-024-03056-x. Respir Res. 2024. PMID: 39709425 Free PMC article. Review.
-
Development of a machine learning model and a web application for predicting neurological outcome at hospital discharge in spinal cord injury patients.Spine J. 2025 Jul;25(7):1483-1493. doi: 10.1016/j.spinee.2025.01.005. Epub 2025 Jan 31. Spine J. 2025. PMID: 39894282
References
-
- WHO Spinal Cord Injury. 2023. [(accessed on 15 April 2025)]. Available online: https://www.who.int/news-room/fact-sheets/detail/spinal-cord-injury.
-
- National Spinal Cord Injury Statistical Center . Traumatic Spinal Cord Injury Facts and Figures at a Glance. University of Alabama at Birmingham; Birmingham, AL, USA: 2024. [(accessed on 15 May 2024)]. Available online: https://sci-bc.ca/wp-content/uploads/2024/05/us-sci-facts-and-stats-1.pdf.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

