Myricetin Amplifies Glucose-Stimulated Insulin Secretion via the cAMP-PKA-Epac-2 Signaling Cascade
- PMID: 40564164
- PMCID: PMC12190580
- DOI: 10.3390/biomedicines13061447
Myricetin Amplifies Glucose-Stimulated Insulin Secretion via the cAMP-PKA-Epac-2 Signaling Cascade
Abstract
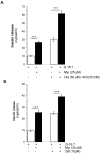
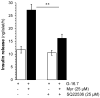
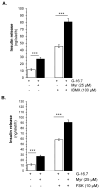
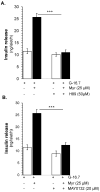
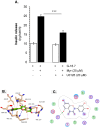
Aim: Myricetin, a natural bioflavonoid, is reported as an anti-diabetic agent since it possesses the ability to inhibit α-glucosidase activity, stimulate insulin action and secretion, manage ROS, and prevent diabetes complications. Myricetin was identified as a new insulin secretagogue that enhances glucose-stimulated insulin secretion and seems like a better antidiabetic drug candidate. Here, we explored the insulinotropic mechanism(s) of myricetin in vitro in mice islets and in silico. Methods: Size-matched pancreatic islets were divided into groups and incubated in the presence or absence of myricetin and agonists/antagonists of major insulin signaling pathways. The secreted insulin was measured by ELISA. Molecular docking studies were performed with the key player of insulin secretory pathways. Results: Myricetin dose-dependently enhanced insulin secretion in isolated mice islets, and its insulinotropic effect was exerted at high glucose concentrations distinctly different from glibenclamide. Myricetin-induced insulin secretion was significantly inhibited using the diazoxide. Furthermore, myricetin amplified glucose-induced insulin secretion in depolarized and glibenclamide-treated islets. Myricetin showed an additive effect with forskolin- and IBMX-induced insulin secretion. Interestingly, H89, a PKA inhibitor, and MAY0132, an Epac-2 inhibitor, significantly inhibited myricetin-induced insulin secretion. The in silico molecular docking studies further validated these in vitro findings in isolated pancreatic islets. Conclusions: Myricetin, a potential natural insulin secretagogue, amplifies glucose-induced insulin secretion via the cAMP-PKA-Epac-2 signaling pathway.
Keywords: antidiabetic drugs; insulin secretagogues; insulin secretion; myricetin; sulfonylurea.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Apigenin potentiates glucose-stimulated insulin secretion through the PKA-MEK kinase signaling pathway independent of K-ATP channels.Biomed Pharmacother. 2024 Aug;177:116986. doi: 10.1016/j.biopha.2024.116986. Epub 2024 Jun 20. Biomed Pharmacother. 2024. PMID: 38906017
-
Insulin secretagogues for prevention or delay of type 2 diabetes mellitus and its associated complications in persons at increased risk for the development of type 2 diabetes mellitus.Cochrane Database Syst Rev. 2016 Oct 17;10(10):CD012151. doi: 10.1002/14651858.CD012151.pub2. Cochrane Database Syst Rev. 2016. PMID: 27749986 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Screening for hyperglycaemia in pregnancy: a rapid update for the National Screening Committee.Health Technol Assess. 2010 Sep;14(45):1-183. doi: 10.3310/hta14450. Health Technol Assess. 2010. PMID: 20868615
References
-
- Bouchi R., Kondo T., Ohta Y., Goto A., Tanaka D., Satoh H., Yabe D., Nishimura R., Harada N., Kamiya H., et al. A consensus statement from the Japan Diabetes Society: A proposed algorithm for pharmacotherapy in people with type 2 diabetes. J. Diabetes Investig. 2023;14:151–164. doi: 10.1111/jdi.13960. - DOI - PMC - PubMed
-
- Mohajan D., Mohajan H.K. Oral Hypoglycaemic Agents: Non-Insulin Medications for Type 2 Diabetes Patients. Innov. Sci. Technol. 2024;3:23–31. doi: 10.56397/IST.2024.01.04. - DOI
LinkOut - more resources
Full Text Sources

