Effect of Cryodiluent and Time of Glycerol Addition on Cryopreservation and In Vitro Fertilization of Domestic Cat Epididymal Spermatozoa
- PMID: 40564232
- PMCID: PMC12189863
- DOI: 10.3390/ani15121680
Effect of Cryodiluent and Time of Glycerol Addition on Cryopreservation and In Vitro Fertilization of Domestic Cat Epididymal Spermatozoa
Abstract
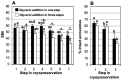
Sperm cryopreservation and assisted reproduction are powerful tools for the conservation of endangered species. The domestic cat has been a useful model for studying wild felid reproductive biology due to the limited availability of endangered individuals for experimental research. Here, we investigate the effect of cryodiluents (TEST vs. Biladyl) and the timing of glycerol addition (before vs. after refrigeration, in one vs. three steps, respectively) on post-thaw sperm quality (motility, acrosome integrity) and their subsequent in vitro fertilization (IVF) ability with homologous oocytes. The results showed no statistically significant differences in sperm traits when samples were cryopreserved in TEST or Biladyl, or when glycerol was added in one or three steps. Motile sperm and intact acrosomes were significantly correlated before and after cryopreservation, indicating consistent relationships in fresh and thawed samples. The use of Biladyl significantly reduced IVF rates after cryopreservation compared to fresh sperm. Cryopreservation in TEST led to IVF rates that were not significantly different from those of fresh sperm. Using swim-up after thawing, or adding 1 mM pentoxifylline, did not enhance IVF results. Overall, a TEST cryodiluent with 4% glycerol added in one step is a reliable option for preserving epididymal cat spermatozoa.
Keywords: cryodiluent; cryopreservation; glycerol; in vitro fertilization; pentoxifylline; sperm; swim-up.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Wildt D.E., Rall W.F., Crister J.K., Monfort S.L., Seal U.S. Genome Resource Banks Living collections for biodiversity conservation. Bioscience. 1997;47:689–698. doi: 10.2307/1313209. - DOI
-
- Roldan E.R.S., Garde J.J. Biotecnología de la reproducción y conservación de especies en peligro de extinción. In: Gomendio M., editor. Los Retos Medioambientales del siglo XXI. La Conservación de la Biodiversidad en España. CSIC-FBBVA; Madrid, Spain: 2004. pp. 283–303.
-
- Swanson W.F., Stoops M.A., Magarey G.M., Herrick J.R. Sperm cryopreservation in endangered felids: Developing linkage of in situ-ex situ populations. In: Roldan E.R.S., Gomendio M., editors. Spermatology. Nottingham University Press; Nottingham, UK: 2007. pp. 417–432. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

