Microsporidia in Rodents- Mus musculus, Rattus norvegicus, and Rattus rattus-A Public Health Concern in the Canary Islands, Spain
- PMID: 40564247
- PMCID: PMC12189156
- DOI: 10.3390/ani15121695
Microsporidia in Rodents- Mus musculus, Rattus norvegicus, and Rattus rattus-A Public Health Concern in the Canary Islands, Spain
Abstract
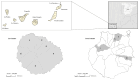
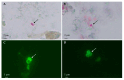
Rodents are recognized as reservoirs of a wide range of pathogens, including microsporidia. The presence of microsporidia in the environment of mainland Spain and its islands has become increasingly known, as the number of studies has multiplied over time. The present study was conducted to determine the occurrence and diversity of microsporidia in three rodent species (Mus musculus, Rattus norvegicus, and Rattus rattus) in the Canary Islands, Spain. Ninety-three fecal samples were obtained from wild rodents on La Gomera and Gran Canaria Islands. Each sample was tested using Weber's modified trichrome staining and immunofluorescence antibody tests (IFATs) against the Encephalitozoon genus and Enterocytozoon bieneusi. The microscopy-positive samples were subsequently analyzed using a nested polymerase chain reaction (PCR) followed by Sanger sequencing. The staining technique showed 38.7% (36/93) positivity, whereas the IFATs for Encephalitozoon spp. and Ent. bieneusi revealed 3.2% (3/93) and 6.5% (6/93) positivity, respectively. Finally, the nested PCR and nucleotide sequence analysis confirmed a 9.7% (9/93) occurrence of Ent. bieneusi and 17.2% occurrence (16/93) of different undetermined microsporidia species, whereas no Encephalitozoon spp. were detected. Seven different Ent. bieneusi genotypes were detected as follows: three known (AAE1, D, and SBM1) and four novel (GRE1, GRE2, LGE1, and LGE2), all of which belonged to Group 1. The results demonstrate, for the first time, that microsporidia are present in the rodent populations of the Canary Islands. Further studies are needed to determine the impact of the presence of microsporidia in rodents on the zoonotic transmission of these parasites.
Keywords: Canary Islands; Encephalitozoon spp.; Enterocytozoon bieneusi; microsporidia; rodents; zoonotic potential.
Conflict of interest statement
Authors Néstor Abreu-Acosta and Estefanía Abreu-Yanes were employed by the company Nertalab S.L. company. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
High prevalence of Enterocytozoon bieneusi (microsporidia) in asymptomatic schoolchildren, Zambia.Med Mycol. 2025 Jul 2;63(7):myaf065. doi: 10.1093/mmy/myaf065. Med Mycol. 2025. PMID: 40693962
-
A Systematic Review and Meta-analysis on the Global Molecular Epidemiology of Microsporidia Infection Among Rodents: A Serious Threat to Public Health.Acta Parasitol. 2022 Mar;67(1):18-30. doi: 10.1007/s11686-021-00447-8. Epub 2021 Jun 27. Acta Parasitol. 2022. PMID: 34176043
-
Genetic Diversity of Enterocytozoon bieneusi in Diarrheic Shelter Dogs in Romania: First Molecular and Phylogenetic Evidence.Pathogens. 2025 Jun 27;14(7):641. doi: 10.3390/pathogens14070641. Pathogens. 2025. PMID: 40732689 Free PMC article.
-
Global molecular epidemiology of microsporidia in pigs and wild boars with emphasis on Enterocytozoon bieneusi: A systematic review and meta-analysis.Vet Med Sci. 2022 May;8(3):1126-1136. doi: 10.1002/vms3.751. Epub 2022 Feb 3. Vet Med Sci. 2022. PMID: 35113502 Free PMC article.
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
References
Grants and funding
- ProID2021010013/Consejería de Economía, Industria, Comercio y Conocimiento (Gobierno de Canarias)
- ProID2021010013/FEDER-DSE Canarias 2014-2020, "Programa de Apoyo a la Investigación María del Carmen Betencourt y Molina"
- "Estudio de patógenos en aves migratorias y en especies exóticas en un escenario de cambio climático"/Consejería de Transición Ecológica, Lucha contra el Cambio Climático y Planificación Territorial (Gobierno de Canarias)
- VII Convocatoria de Ayudas a la Movilidad CEINDO 22-23/CEINDO
- FPI predoctoral scholarship (TESIS2021010056) and (TESIS2022010038)/Agencia Canaria de Investigación, Innovación y Sociedad de la Información de la Consejería de Economía, Conocimiento y Empleo
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

