Effect of Potassium-Magnesium Sulfate on Intestinal Dissociation and Absorption Rate, Immune Function, and Expression of NLRP3 Inflammasome, Aquaporins and Ion Channels in Weaned Piglets
- PMID: 40564302
- PMCID: PMC12189249
- DOI: 10.3390/ani15121751
Effect of Potassium-Magnesium Sulfate on Intestinal Dissociation and Absorption Rate, Immune Function, and Expression of NLRP3 Inflammasome, Aquaporins and Ion Channels in Weaned Piglets
Abstract
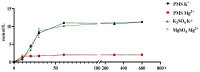
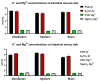
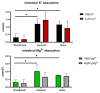
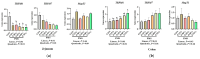
This study investigated the effects of potassium magnesium sulfate (PMS) on intestinal dissociation and absorption rate, immune function, and expression of the NOD-like receptor thermal domain-associated protein 3 (NLRP3) inflammasome, aquaporins (AQPs), and potassium and magnesium ion channels in weaned piglets. Experiment 1 involved the assessment of the dissociation rate of PMS in pig digestive fluid and the absorption rate of PMS in the small intestine using an Ussing chamber in vitro. In Experiment 2, 216 healthy 21-day-old weaned piglets were selected and randomly assigned to six groups (0%, 0.15%, 0.30%, 0.45%, 0.60%, and 0.75% PMS), with each group 6 replicates of six piglets per replicate. The in vitro Ussing chamber results indicated that the absorption of K+ and Mg2+ in the jejunum and ileum was significantly higher than that in the duodenum (p < 0.05). The in vivo study demonstrated that the addition of PMS resulted in a linear increase in serum K+, IgG, and interleukin (IL)-2 levels while simultaneously reducing serum IL-1β levels (p < 0.05). Dietary PMS significantly elevated serum IL-10 and Mg2+ levels in feces (p < 0.05). Furthermore, supplementation with 0.60% or 0.75% PMS significantly downregulated the mRNA expression of NLRP3 in the jejunum (p < 0.05). Dietary PMS supplementation linearly reduced the mRNA expression levels of cysteine protease 1 (Caspase-1) and IL-1β in both the jejunum and colon as well as the mRNA expression levels of two-pore domain channel subfamily K member 5 (KCNK5) in these regions (p < 0.05). Notably, supplementation with 0.15% PMS significantly decreased the mRNA expression of transient receptor potential channel 6 (TRPM6) in the jejunum and significantly increased the expression of TRPM6 in the colon (p < 0.05). Dietary addition of 0.45% and 0.60% PMS significantly increased the mRNA expression of aquaporin 3 (AQP3) in the colon (p < 0.05), whereas 0.75% PMS significantly increased the mRNA expression of aquaporin 8 (AQP8) in both the jejunum and colon. Moreover, the expression levels of AQP3 and AQP8 were significantly negatively correlated with the diarrhea rate observed between days 29 and 42. In conclusion, dietary PMS supplementation improved immune function, inhibited the activation of intestinal NLRP3, and modulated the expression of water and ion channels in weaned piglets, thereby contributing to the maintenance of intestinal water and ion homeostasis, which could potentially alleviate post-weaning diarrhea in piglets. The recommended supplemental level of PMS in the corn-soybean basal diet for weaned piglets is 0.30%.
Keywords: NLPR3; aquaporins; ion channel; magnesium potassium sulfate; weaned piglets.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources

