Effects of Different Rearing Methods on the Intestinal Morphology, Intestinal Metabolites, and Gut Microbiota of Lueyang Black-Bone Chickens
- PMID: 40564310
- PMCID: PMC12189338
- DOI: 10.3390/ani15121758
Effects of Different Rearing Methods on the Intestinal Morphology, Intestinal Metabolites, and Gut Microbiota of Lueyang Black-Bone Chickens
Abstract
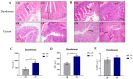
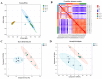
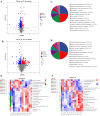
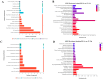
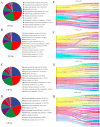
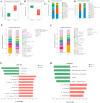
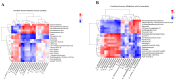
The Lueyang black-bone chicken represents a distinct indigenous avian breed native to China and it is a slow-growing broiler breed. The gut, whose primary function is to digest food and absorb nutrients, is also home to a large and diverse microbial community. The intestinal morphology, intestinal metabolites, and gut microbiota are critical determinants of nutrient utilization efficiency and immune health in poultry. This study investigates the impact of two distinct rearing modalities-cage-raised (CR) and cage-free (CF)-on the intestinal morphology, intestinal metabolites, and gut microbiota of the duodenum and cecum in Lueyang black-bone chickens. Additionally, we have integrated metabolomics and microbiome analyses. Morphological assessments revealed that, in comparison to the CR group, the CF group exhibited a significant increase in duodenal villi height (VH) and crypt depth (CD) (p < 0.01). Furthermore, there was a notable increase in the number of intestinal inflammatory cells within the CF group. Non-targeted metabolomics indicated an upregulation of omega-3 series polyunsaturated fatty acids and bile acid metabolites in the CR group. Conversely, the CF group demonstrated significantly elevated levels of lysophosphatidylcholine (LPC) and phosphatidylcholine (PE) in the intestine. Microbiome analysis revealed that in the duodenum, beneficial bacteria (e.g., Lactobacillus) were the dominant genera in the CF group, while the Bacteroides predominate in the CR group. Correlation analyses indicated a positive association between LPC levels and the presence of eight bacterial genera, including Ureaplasma. The omega-3 series polyunsaturated fatty acids were positively correlated with three bacterial genera, such as Flavobacterium. Notably, bile acid metabolites exhibited a significant positive correlation with Rikenellaceae_RC9_gut_group. In conclusion, this study provides novel insights into how rearing methods influence intestinal morphology, intestinal metabolites, and gut microbiota, offering a new perspective for the scientific management of poultry with the premise of ensuring animal health and welfare.
Keywords: Lueyang black-bone chicken; gut microbiota; intestinal metabolites; intestinal morphology; rearing method.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures
References
LinkOut - more resources
Full Text Sources

