Mulberry (Morus alba) Twig and Leaf Extracts Ameliorate Obesity-Related Metabolic Disorders via Gut Microbiota Modulation in High-Fat Diet-Fed Mice
- PMID: 40564320
- PMCID: PMC12189312
- DOI: 10.3390/ani15121768
Mulberry (Morus alba) Twig and Leaf Extracts Ameliorate Obesity-Related Metabolic Disorders via Gut Microbiota Modulation in High-Fat Diet-Fed Mice
Abstract
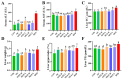
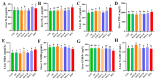
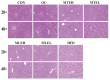
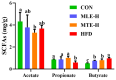
Mulberry (Morus alba) twigs and leaves, rich in flavonoids, polyphenols, polysaccharides, and alkaloids with multi-target regulatory properties on glucose/lipid metabolism, were evaluated for their anti-obesity effects using methanol-extracted twigs (MTE) and aqueous-extracted leaves (MLE) in high-fat diet (HFD)-induced obese mice. Both extracts significantly ameliorated obesity-related metabolic dysregulation, as evidenced by attenuated body weight gain, visceral fat accumulation, serum lipid profiles, homeostatic model assessment of insulin resistance (HOMA-IR), and hepatic inflammation compared to HFD controls (p < 0.05). Concurrently, MTE and MLE enhanced systemic antioxidant capacity and elevated high-density lipoprotein cholesterol (HDL-C) levels. Notably, high-dose MTE (MTEH, 1000 mg/kg) markedly reduced perirenal adiposity while increasing brown adipose tissue mass (p < 0.05). Mechanistic investigations revealed that MTEH reshaped gut microbiota composition by suppressing Firmicutes and Enterococcus, while enriching beneficial Faecalibaculum and Bifidobacterium spp. (p < 0.05). Furthermore, cecal short-chain fatty acid (SCFA) profiling demonstrated MTEH and MLEH-mediated metabolic reprogramming, characterized by increased propionic acid and decreased butyric acid, suggesting microbiota-dependent modulation of host energy metabolism. These findings collectively highlight the potential of mulberry extracts as multi-targeted nutraceuticals for obesity intervention via gut microbiota-SCFA axis regulation.
Keywords: glycolipid metabolism; gut microbiology; mulberry extract; obesity.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures








References
-
- Xu J., Wu T., Lam S.M., Shui G., Yang S., Wang Y., Tao C. Heterogeneity of Intramuscular, Intermuscular, and Subcutaneous Fat in Laiwu Pigs: Insights from Targeted Lipidomics and Transcriptomics. Agriculture. 2024;14:658. doi: 10.3390/agriculture14050658. - DOI
-
- Velázquez K.T., Enos R.T., Bader J.E., Sougiannis A.T., Carson M.S., Chatzistamou I., Carson J.A., Nagarkatti P.S., Nagarkatti M., Murphy E.A. Prolonged high-fat-diet feeding promotes non-alcoholic fatty liver disease and alters gut microbiota in mice. World J. Hepatol. 2019;11:619–637. doi: 10.4254/wjh.v11.i8.619. - DOI - PMC - PubMed
Grants and funding
- Z222020001/Shiquan Silkworm and Mulberry Demonstration Base
- K3010724016/National Silkworm and Mulberry Industry Technology System Comprehensive Experimental Station of Ecological Mulberry in North Shaanxi Province
- K3031224100/R&D and Experimentation of Key Supporting Technologies for Highly Efficient Silkworm and Mulberry Farming and Rearing (Silkworm and Mulberry)
LinkOut - more resources
Full Text Sources
Miscellaneous

