Enhanced Aerosol Containment Performance of a Negative Pressure Hood with an Aerodynamic Cap Design: Multi-Method Validation Using CFD, PAO Particles, and Microbial Testing
- PMID: 40564440
- PMCID: PMC12189663
- DOI: 10.3390/bioengineering12060624
Enhanced Aerosol Containment Performance of a Negative Pressure Hood with an Aerodynamic Cap Design: Multi-Method Validation Using CFD, PAO Particles, and Microbial Testing
Abstract
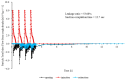
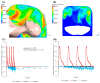
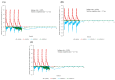
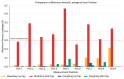
Healthcare providers performing aerosol-generating procedures (AGPs) face significant infection risks, emphasizing the critical need for effective aerosol containment systems. In this study, we developed and validated a negative pressure chamber enhanced with an innovative aerodynamic cap structure designed to optimize aerosol containment. Initially, computational fluid dynamics (CFD) simulations were performed to evaluate multiple structural improvement ideas, including air curtains, bidirectional suction, and aerodynamic cap structures. Among these, the aerodynamic cap was selected due to its superior predicted containment performance, practical feasibility, and cost-effectiveness. The CFD analyses employed realistic transient boundary conditions, precise turbulence modeling using the shear stress transport (SST) k-ω model, and detailed droplet evaporation dynamics under realistic humidity conditions. A full-scale prototype incorporating the selected aerodynamic cap was fabricated and evaluated using physical polyalphaolefin (PAO) particle leakage tests and biological aerosol validation with aerosolized Bacillus subtilis. For the physical leakage tests, the chamber opening was divided into nine sections, and the aerosol dispersion was tested in three distinct directions: ceiling-directed, toward the suction hole, and opposite the suction hole. These tests demonstrated significantly stabilized airflow and substantial reductions in aerosol leakage, consistently maintaining containment levels below the critical threshold of 0.3%, especially under transient coughing conditions. The biological aerosol experiments, conducted in a simulated emergency department environment, involved aerosolizing bacteria continuously for one hour. The results confirmed the effectiveness of the aerodynamic cap structure in achieving at least a one millionth (10-6) reduction in the aerosolized bacterial leakage compared to the control conditions. These findings highlight the importance and effectiveness of advanced CFD modeling methodologies in accurately predicting aerosol dispersion and improving containment strategies. Although further studies assessing the structural durability, long-term operational ease, and effectiveness against pathogenic microorganisms are required, the aerodynamic cap structure presents a promising, clinically practical infection control solution for widespread implementation during aerosol-generating medical procedures.
Keywords: aerosol transmission; computational fluid dynamics; intubation; respiratory infection.
Conflict of interest statement
Authors Kisub Sung and Jung-Woo Lee were employed by the company SS-ENG Co., Ltd. The funding sponsor, “The Ministry of Health and Welfare, Republic of Korea”, had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







Similar articles
-
Enhancing vehicle performance through the application of airfoils as spoilers with movable trailing edge.F1000Res. 2025 Jun 9;14:469. doi: 10.12688/f1000research.160307.2. eCollection 2025. F1000Res. 2025. PMID: 40529636 Free PMC article.
-
Topotecan, pegylated liposomal doxorubicin hydrochloride and paclitaxel for second-line or subsequent treatment of advanced ovarian cancer: a systematic review and economic evaluation.Health Technol Assess. 2006 Mar;10(9):1-132. iii-iv. doi: 10.3310/hta10090. Health Technol Assess. 2006. PMID: 16545208
-
Evaluating the effectiveness of a novel cost-effective aerosol containment chamber for high-risk autopsies: a pilot study.Sci Rep. 2025 Jul 2;15(1):22858. doi: 10.1038/s41598-025-05271-y. Sci Rep. 2025. PMID: 40594082 Free PMC article.
-
Measures implemented in the school setting to contain the COVID-19 pandemic.Cochrane Database Syst Rev. 2022 Jan 17;1(1):CD015029. doi: 10.1002/14651858.CD015029. Cochrane Database Syst Rev. 2022. Update in: Cochrane Database Syst Rev. 2024 May 2;5:CD015029. doi: 10.1002/14651858.CD015029.pub2. PMID: 35037252 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
References
-
- World Health Organization . Infection Prevention and Control During Health Care When Coronavirus Disease (COVID-19) Is Suspected or Confirmed. WHO; Geneva, Switzerland: 2021.
-
- Centers for Disease Control and Prevention . Prevention Interim Infection Prevention and Control Recommendations for Healthcare Personnel During the Coronavirus Disease 2019 (COVID-19) Pandemic. Centers for Disease Control and Prevention; Atlanta, GA, USA: 2022.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

