Functionally Isolated Sarcoplasmic Reticulum in Cardiomyocytes: Experimental and Mathematical Models
- PMID: 40564443
- PMCID: PMC12189891
- DOI: 10.3390/bioengineering12060627
Functionally Isolated Sarcoplasmic Reticulum in Cardiomyocytes: Experimental and Mathematical Models
Abstract
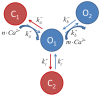
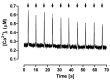
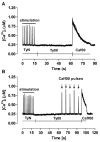
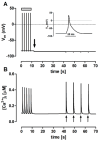
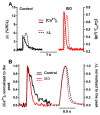
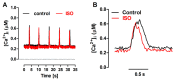
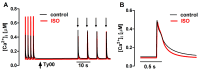
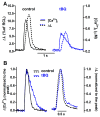
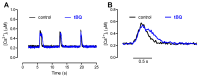
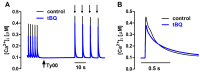
The interaction among the various Ca2+ transporters complicates the assessment of isolated systems in an intact cell. This article proposes the functionally isolated SR model (FISRM), a hybrid (experimental and mathematical) approach to study Ca2+ cycling between the cytosol and the sarcoplasmic reticulum (SR), the main source of Ca2+ for contraction in mammalian cardiomyocytes. In FISRM, the main transmembrane Ca2+ transport pathways are eliminated by using a Na+, Ca2+-free extracellular medium, and SR Ca2+ release is elicited by a train of brief caffeine pulses. Two compounds that exert opposite effects on the SR Ca2+ uptake were characterized by this approach in isolated rat ventricular cardiomyocytes. The experimental FISRM was simulated with a simple mathematical model of Ca2+ fluxes across the SR membrane, based on a previous model adapted to the present conditions. To a fair extent, the theoretical model could reproduce the experimental results, and confirm the main assumption of the experimental model: that the only relevant Ca2+ fluxes occur across the SR membrane. Thus, the FISRM seems to be a valuable framework to investigate the SR Ca2+ transport in intact cardiomyocytes under physiological and pathophysiological conditions, and to test therapeutic approaches targeting SR proteins.
Keywords: 2,5-di-tert-butylhydroquinone; Ca2+ transport; caffeine; cardiomyocytes; mathematical modeling; sarcoplasmic reticulum; β-adrenergic stimulation.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of this study, in the collection, analyses, or interpretation of data; in the writing of this manuscript; or in the decision to publish the results.
Figures
Similar articles
-
Eliciting adverse effects data from participants in clinical trials.Cochrane Database Syst Rev. 2018 Jan 16;1(1):MR000039. doi: 10.1002/14651858.MR000039.pub2. Cochrane Database Syst Rev. 2018. PMID: 29372930 Free PMC article.
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
-
Effects of 8 Weeks of Moderate- or High-Volume Strength Training on Sarcoplasmic Reticulum Ca2+ Handling in Elite Female and Male Rowers.Scand J Med Sci Sports. 2025 Jan;35(1):e70017. doi: 10.1111/sms.70017. Scand J Med Sci Sports. 2025. PMID: 39831408 Free PMC article. Clinical Trial.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
The measurement of collaboration within healthcare settings: a systematic review of measurement properties of instruments.JBI Database System Rev Implement Rep. 2016 Apr;14(4):138-97. doi: 10.11124/JBISRIR-2016-2159. JBI Database System Rev Implement Rep. 2016. PMID: 27532315
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

