The Risks Associated with Inhalation Exposure to Cosmetics and Potential for Assessment Using Lung Organoids
- PMID: 40564468
- PMCID: PMC12189234
- DOI: 10.3390/bioengineering12060652
The Risks Associated with Inhalation Exposure to Cosmetics and Potential for Assessment Using Lung Organoids
Abstract
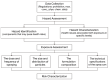
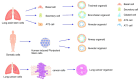
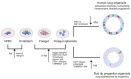
This review addresses the exposure risks associated with the inhalation of aerosolized cosmetic products and explores the utility of lung organoids in assessing these risks. Aerosolized cosmetics such as sprays pose potential health hazards through inhalation, necessitating a thorough evaluation of exposure levels. Traditional methods for assessing inhalation risks have limitations, prompting the exploration of more sophisticated models. Lung organoids, three-dimensional structures derived from stem cells, offer a biologically relevant model for studying lung responses to inhaled substances. This review discusses the construction of lung organoids, their characteristics, and the advantages that they provide over conventional models. Furthermore, it examines existing studies that have employed lung organoids to evaluate the effects of cosmetic inhalation exposure, highlighting the potential of this approach to enhance the safety assessments of cosmetic products. We aim to establish lung organoids as a reliable tool for future research, ensuring the safety and regulatory compliance of cosmetics.
Keywords: cosmetics; inhalation exposure; lung organoids; risk assessment.
Conflict of interest statement
Authors Xin Luo and Qi Xiang were employed by the company Biopharmaceutical R&D Center of Jinan University Co., Ltd. Author Rong Hu was employed by the company Shenzhen Hujia Technology Co., Ltd. Author Lifeng Tang was employed by the company Guangzhou Xika Technology Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
How lived experiences of illness trajectories, burdens of treatment, and social inequalities shape service user and caregiver participation in health and social care: a theory-informed qualitative evidence synthesis.Health Soc Care Deliv Res. 2025 Jun;13(24):1-120. doi: 10.3310/HGTQ8159. Health Soc Care Deliv Res. 2025. PMID: 40548558
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
Comparison of the effectiveness of inhaler devices in asthma and chronic obstructive airways disease: a systematic review of the literature.Health Technol Assess. 2001;5(26):1-149. doi: 10.3310/hta5260. Health Technol Assess. 2001. PMID: 11701099
-
Individual-level interventions to reduce personal exposure to outdoor air pollution and their effects on people with long-term respiratory conditions.Cochrane Database Syst Rev. 2021 Aug 9;8(8):CD013441. doi: 10.1002/14651858.CD013441.pub2. Cochrane Database Syst Rev. 2021. PMID: 34368949 Free PMC article.
References
-
- Lyu J., Jiang Y., Liu J., Wang S., Diao Q. Review on the Mechanisms of Skin Adverse Reactions of Cosmetics. J. Clin. Dermatol. 2023;52:240–243. doi: 10.16761/j.cnki.1000-4963.2023.04.016. - DOI
-
- Laser Cosmetology Group, Medical Aesthetics and Cosmetology Branch of Chinese Medical Association. Skin Care Product and Material Group, Committee on Skin Disease and Cosmetic Dermatology, China Association of Medical Equipment. Laser Group, Cosmetic and Plastic Surgeon Branch of Chinese Medical Doctor Association. Cosmetic Laser Group, Chinese Society of Dermatology Guidelines for the Diagnosis and Treatment of Adverse Skin Reactions to Cosmetics (2024 Edition) Chin. J. Dermatol. 2024;57:485–492. doi: 10.35541/cjd.20230674. - DOI
-
- Leng W., He X., Wang W., Luo Z. Clinical Characteristics of 1089 Cases with Cosmetic Dermatoses in Lanzhou Area. J. Clin. Dermatol. 2024;53:449–453.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources