Long-Term Efficacy and Safety of Leuprorelin Treatment in Children with Central Precocious Puberty: A Systematic Review and Meta-Analysis
- PMID: 40564670
- PMCID: PMC12191920
- DOI: 10.3390/children12060712
Long-Term Efficacy and Safety of Leuprorelin Treatment in Children with Central Precocious Puberty: A Systematic Review and Meta-Analysis
Abstract
Background: As the first approved GnRH agonist, leuprorelin is distinguished by its broad application in managing central precocious puberty (CPP). Despite the extensive use of leuprorelin in CPP management, uncertainties still persist regarding its long-term efficacy and safety. We conducted a systematic review and meta-analysis to assess the long-term efficacy and safety of leuprorelin treatment in children with CPP. Methods: We conducted electronic searches in PubMed, Embase, and the Cochrane Library up until 15 November 2023. All relevant studies concerning leuprorelin treatment in children with CPP were included. Results: The final adult height of children with CPP eventually reached the target height, with a significant difference of MD: 1.75 cm (95% CI: 0.46-3.03). The MD in BMI standard deviation score between baseline and post-leuprorelin treatment was -0.03 (95% CI: -0.28-0.22). For the onset of menstrual puberty, the MD between children with CPP who received leuprorelin treatment and those who did not was 0.73 years latency (95% CI: -0.74-2.20) without significant difference. The timing of menstrual puberty of the leuprorelin-treated group was 15.83 months (95% CI: 11.62-20.03) after the discontinuation of leuprorelin treatment. The proportion of menstrual regularity was 85% (95% CI: 75-91%), and the average incidence rate of polycystic ovary syndrome (PCOS) was 8% (95% CI: 3-22%) for children with CPP that treated with leuprorelin. Conclusions: Leuprorelin treatment does not affect BMI or the onset of menstrual puberty in the long term, but has positive effects on adult height for children with CPP. Moreover, no severe adverse events related to leuprorelin treatment were observed.
Keywords: central precocious puberty; children; leuprorelin; long-term; meta-analysis.
Conflict of interest statement
Ling Hou, Yanqin Ying, Feng Ye, Cai Zhang: Members of advisory council/committees for Takeda, speakers for Takeda, research funding from Takeda. Xiaoping Luo: Member of advisory council/committees for GenSci, Amoytop, Novo Nordisk, Takeda, Lumos, Sanofi, Medtronic, Ipsen, Visen, Kyowa Kirin. Research funding from GenSci, Amoytop, Novo Nordisk, Takeda, Lumos, Sanofi, Medtronic, Ipsen, Visen, Kyowa Kirin. Speaker for GenSci, Novo Nordisk, Visen.
Figures

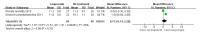



References
-
- Subspecialty Group of Endocrinology, Heredity and Metabolism, Society of Pediatrics, Chinese Medical Association. Editorial Board of Chinese Journal of Pediatrics Consensus on the diagnosis and treatment of central precocious puberty (2015) Chin. J. Pediatr. 2015;53:412–418. - PubMed
-
- Subspecialty Group of Endocrinology, Heredity and Metabolism, Society of Pediatrics, Chinese Medical Association. Editorial Board of Chinese Journal of Pediatrics Expert consensus on the diagnosis and treatment of central precocious puberty (2022) Chin. J. Pediatr. 2022;61:16–22. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

