Gut Microbiota Differences in Infants with Cow-Milk-Induced Allergic Proctocolitis: A Comparative Cross-Sectional Study
- PMID: 40564692
- PMCID: PMC12191891
- DOI: 10.3390/children12060734
Gut Microbiota Differences in Infants with Cow-Milk-Induced Allergic Proctocolitis: A Comparative Cross-Sectional Study
Abstract
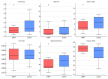
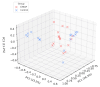
Background: Cow-milk-induced allergic proctocolitis (CMIAP) is a non-IgE-mediated food hypersensitivity that often resolves spontaneously but may predispose infants to IgE-mediated allergies and eosinophilic gastrointestinal disorders. Understanding its pathophysiology is crucial for microbiota-based interventions. Methods: We enrolled 32 exclusively breastfed infants-16 with confirmed cases of CMIAP and 16 age-matched healthy controls. The cohorts were sex-balanced (8 F/8 M), term-born (gestational age ± SD: 40 ± 1.2 vs. 39 ± 1.3 weeks), vaginally delivered, and sampled at a mean age of 2.0 ± 0.44 months (range 1.5-3.0) vs. 2.4 ± 0.66 months (range 1.5-3.5). Faecal samples underwent 16S rRNA gene sequencing on the Illumina NovaSeq platform, with diversity and differential abundance analyses. Results: The maternal dairy intake was similar (total dairy: 250 ± 80 vs. 240 ± 75 mL/day; yoghurt: 2.3 ± 1.0 vs. 2.5 ± 1.2 days/week; p = 0.72). Bray-Curtis dissimilarity assessments revealed distinct microbiota in infants with CMIAP. Infants with CMIAP had a lower abundance of Bifidobacterium (log2FC-2.27; q = 0.022; ANCOM-BC), Collinsella (-29.35; padj < 0.0001; DESeq2), and Limosilactobacillus (-8.01; padj = 0.0285; DESeq2; q < 0.0001; ANCOM-BC) compared with controls. In contrast, Hungatella (+24.99; padj < 0.0001; DESeq2), Veillonella (+4.73; padj = 0.0221; DESeq2), Citrobacter (+10.44; padj = 0.0124; DESeq2), and Ruminococcus gnavus (+2.69; q < 0.0001; ANCOM-BC) were more abundant in the CMIAP group. Conclusions: Infants with CMIAP exhibit gut dysbiosis, which is characterised by the depletion of beneficial commensals and the enrichment of potential pathogens, independent of maternal dairy intake. Further studies should establish whether these microbiota alterations are causal or consequential in CMIAP.
Keywords: cow-milk-induced allergic proctocolitis; faecal microbiota; infants; microbial diversity.
Conflict of interest statement
All authors declare that there are no financial or non-financial interests that are directly or indirectly related to the work submitted for publication.
Figures
References
-
- Carucci L., Nocerino R., Coppola S., Bedogni G., Capasso P., Giglio V., Canani R. Factors Influencing the Natural History of Non-IgE-Mediated Gastrointestinal Food Allergies in Paediatric Age: A Prospective Multicentre Cohort Study. BMJ Paediatr. Open. 2025;9:e003203. doi: 10.1136/bmjpo-2024-003203. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

