Eosinophilic Cells as a Distinct Morphological Feature in BRAFV600E-Mutated Ovarian Serous Borderline Tumors
- PMID: 40564801
- PMCID: PMC12192365
- DOI: 10.3390/diagnostics15121479
Eosinophilic Cells as a Distinct Morphological Feature in BRAFV600E-Mutated Ovarian Serous Borderline Tumors
Abstract
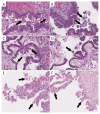
Background/Objectives: According to recent reports, the BRAFV600E mutation in serous borderline tumors (SBTs) plays a protective role against progression to low-grade serous carcinoma through oncogene-induced senescence. One consequence of this is the appearance of eosinophilic cells (ECs). The aim of the current study was to determine the interobserver reproducibility of ECs and their predictive significance for the detection of the BRAFV600E mutation in SBTs. Methods: The study was conducted using 63 cases of ovarian SBTs. Three gynecological pathologists, blinded to each tumor's mutation status, assessed the presence of ECs. Immunohistochemical staining with p16 and Ki-67 was performed to validate ECs. Mutational analysis was carried out using targeted NGS. Results: Genetic analysis revealed 30 BRAF-mutated, 1 NRAS-mutated, and 9 KRAS-mutated SBTs. ECs were identified by the majority of pathologists (two or three) in 78% of the BRAFV600E-mutated and 11% of the wild-type tumors with other mutations (p < 0.0001). The interobserver reproducibility of the presence of ECs was substantial (κ = 0.66). ECs validated with p16/Ki-67 were identified in 92.6% of the BRAFV600E-mutated and in 13.8% of the wild-type tumors with other mutations (p < 0.0001). For the ECs identified by the majority of pathologists, the sensitivity and specificity when predicting the BRAFV600E mutation were 77.8% and 88.9%, respectively. For the ECs validated with p16/Ki-67, the sensitivity and specificity when predicting the BRAFV600E mutation were 95.3% and 90.5%, respectively. Conclusions: Overall, these results suggest that ECs in SBTs have potential association with the BRAFV600E mutation.
Keywords: BRAF; KRAS; NRAS; eosinophilic cells; interobserver reproducibility; oncogene-induced senescence; serous borderline tumor.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Vang R., Davidson B., Kong C.S., Longacre T.A., Malpica A. WHO Classification of Female Genital Tumors. IARC; Lyon, France: 2021. pp. 38–42.
-
- Chui M.H., Xing D., Zeppernick F., Wang Z.Q., Hannibal C.G., Frederiksen K., Kjaer S.K., Cope L., Kurman R.J., Shih I.-M., et al. Clinicopathologic and molecular features of paired cases of metachronous ovarian serous borderline tumor and subsequent serous carcinoma. Am. J. Surg. Pathol. 2019;43:1462–1472. doi: 10.1097/PAS.0000000000001325. - DOI - PMC - PubMed
-
- Hannibal C.G., Vang R., Junge J., Frederiksen K., Kurman R.J., Kjaer S.K. A nationwide study of ovarian serous borderline tumors in Denmark 1978-2002. Risk of recurrence, and development of ovarian serous carcinoma. Gynecol. Oncol. 2017;144:174–180. doi: 10.1016/j.ygyno.2016.11.007. - DOI - PMC - PubMed
-
- Vang R., Hannibal C.G., Junge J., Frederiksen K., Kjaer S.K., Kurman R.J. Long-term behavior of serous borderline tumors subdivided into atypical proliferative tumors and noninvasive low-grade carcinomas: A population-based clinicopathologic study of 942 cases. Am. J. Surg. Pathol. 2017;41:725–737. doi: 10.1097/PAS.0000000000000824. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

