The Problem of Molecular Target Choice for CAR-T Cells in Acute Myeloid Leukemia Therapy
- PMID: 40564892
- PMCID: PMC12192633
- DOI: 10.3390/ijms26125428
The Problem of Molecular Target Choice for CAR-T Cells in Acute Myeloid Leukemia Therapy
Abstract
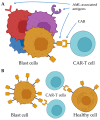
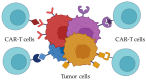
Recently, the chimeric antigen receptor (CAR)-T approach represented a breakthrough in the treatment of B-cell malignancies, encouraging the application of the approach for other hematological diseases, such as acute myeloid leukemia (AML). Heterogeneity and antigen variation in the pathological cell population hinder the choice of molecular targets in the case of AML. In this review, the critical aspects were described that are usually considered when selecting molecular targets for the new CAR genetic constructs. The role of AML-associated antigens in AML progression was covered. In conclusion, we proposed an approach that may allow the elimination of pathological cells in AML more effectively.
Keywords: CAR T; acute myeloid leukemia; target choice.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Ryan C. FDA Approves Obecabtagene Autoleucel for Adults with Relapsed or Refractory B-Cell Precursor Acute Lymphoblastic Leukemia. FDA; Silver Spring, MD, USA: 2024.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

