Hyperthermophilic L-Asparaginase from Thermococcus sibiricus and Its Double Mutant with Increased Activity: Insights into Substrate Specificity and Structure
- PMID: 40564901
- PMCID: PMC12193700
- DOI: 10.3390/ijms26125437
Hyperthermophilic L-Asparaginase from Thermococcus sibiricus and Its Double Mutant with Increased Activity: Insights into Substrate Specificity and Structure
Abstract
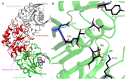
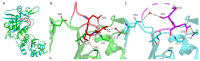
L-asparaginase (L-ASNase) is a key industrial enzyme significant for cancer therapy and the food industry for reducing dietary acrylamide. The hyperthermophilic L-ASNase from Thermococcus sibiricus (TsAI) was previously shown to exhibit high activity and thermostability and is promising for biotechnology. To gain insights into structure-functional relationships of TsAI, determination of the substrate specificity, kinetic parameters, structural characterization, and molecular docking were performed. TsAI characteristics were compared with the TsAID54G/T56Q mutant, which exhibited increased activity after a double mutation in the substrate-binding region. TsAI and TsAID54G/T56Q were found to display high activity towards D-asparagine-62% and 21% of L-asparaginase activity, respectively-and low L-glutaminase coactivity of ~5%. Restoring the mesophilic-like triad GSQ in the mutant resulted in a two-fold increase in activity towards L-asparagine compared with TsAI. Crystal structures of TsAI forms solved at 1.9 Å resolution revealed that double mesophilic-like mutation increased the flexibility of the loop M51-L57, located in close proximity to the active site. Structural superposition and mutational analysis indicate that mobility of this loop is essential for the activity of thermo-ASNases. Molecular docking, without taking into account the temperature factor, showed that, in contrast to L-asparagine interaction, D-asparagine orientation in the TsAI and TsAID54G/T56Q active sites is similar and not optimal for catalysis. Under real conditions, high temperatures can induce structural changes that reduce L-ASNase discrimination towards D-asparagine. Overall, the obtained structural and biochemical data provide a basis for a more detailed understanding of thermo-ASNase functioning and possibilities to engineer improved variants for future biotechnological application.
Keywords: L-asparaginase; hyperthermophilic enzyme; stereoselectivity; substrate specificity; thermo-L-asparaginase structure.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Zhang Z., Chen Y., Deng P., He Z., Qin F., Chen Q., Wang Z., Pan H., Chen J., Zeng M. Research Progress on Generation, Detection and Inhibition of Multiple Hazards—Acrylamide, 5-Hydroxymethylfurfural, Advanced Glycation End Products, Methylimidazole—In Baked Goods. Food Chem. 2024;431:137152. doi: 10.1016/j.foodchem.2023.137152. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

