Inflammasomes in Cardiovascular Diseases: Current Knowledge and Future Perspectives
- PMID: 40564905
- PMCID: PMC12193278
- DOI: 10.3390/ijms26125439
Inflammasomes in Cardiovascular Diseases: Current Knowledge and Future Perspectives
Abstract
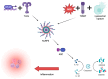
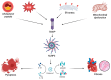
Chronic inflammation is an important contributor to the development of cardiovascular disorders, and inflammasomes, especially the NOD-like receptor protein 3 (NLRP3), are emerging as crucial mediators in this context. Inflammasomes are activated through receptor-mediated danger signals, such as cholesterol crystals and cellular damage products, thereby stimulating the secretion of pro-inflammatory cytokines, which sustains inflammation. This mechanism drives atherosclerosis (via plaque formation and destabilization), heart failure (via fibrotic remodeling), and pericarditis (via exacerbation of pericardial inflammation). Therapeutic approaches seek to block inflammasome activation or their pro-inflammatory pathways. Colchicine, interleukin-1 inhibitors (anakinra, canakinumab), and Sodium-Glucose Transport Protein 2 (SGLT2) inhibitors have a positive impact on cardiovascular inflammation. Various new compounds, such as MCC950, have been described as novel specific inhibitors of NLRP3. Further studies are needed to validate the effectiveness and safety of these treatments. Further elucidating the role of inflammasomes in cardiovascular disease could open the way to achieving more effective therapies, allowing for better management of high-risk cardiovascular patients.
Keywords: NLRP3; atherosclerosis; cardiovascular diseases; heart failure; inflammasomes; pericarditis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

