Comparative Efficacy of Exosomes Derived from Different Mesenchymal Stem Cell Sources in Osteoarthritis Models: An In Vitro and Ex Vivo Analysis
- PMID: 40564912
- PMCID: PMC12193399
- DOI: 10.3390/ijms26125447
Comparative Efficacy of Exosomes Derived from Different Mesenchymal Stem Cell Sources in Osteoarthritis Models: An In Vitro and Ex Vivo Analysis
Abstract
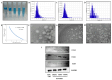
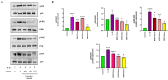
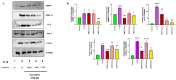
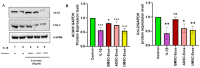
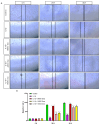
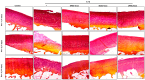
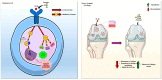
Osteoarthritis (OA) is a prevalent and debilitating joint disorder that affects a substantial proportion of the global population, underscoring the urgent need for therapeutic strategies that extend beyond symptomatic management. Although mesenchymal stem cells (MSCs) have emerged as a promising therapeutic modality, their clinical application remains constrained by several inherent limitations. This study explores a cell-free alternative by investigating the therapeutic potential of exosomes derived from bone marrow (BMSCs), adipose tissue (ADSCs), and umbilical cord (UMSCs) MSCs in mitigating OA pathogenesis, utilizing both in vitro and ex vivo models. Exosomes from each MSC source were isolated and characterized through nanoparticle tracking analysis, transmission electron microscopy, and Western blotting to confirm their identity and purity. Subsequently, their chondroprotective, anti-inflammatory, and regenerative properties were systematically assessed through evaluations of cell viability, expression profiles of inflammatory and chondroprotective markers, and chondrocyte migration assays. The results demonstrate that all three types of MSC-derived exosomes (MSC-Exos) exhibit low cytotoxicity while significantly suppressing proinflammatory markers and enhancing the expression of chondroprotective genes. Notably, BMSC-Exos and UMSC-Exos displayed superior efficacy in attenuating inflammation, promoting cartilage protection, and inhibiting chondrocyte apoptosis. Furthermore, all MSC-Exos markedly enhanced chondrocyte motility, a critical component of cartilage repair. Collectively, these findings support the therapeutic promise of MSC-Exos, particularly those derived from BMSCs and UMSCs, as a targeted, cell-free approach for the treatment of OA compared to ADSCs. By modulating inflammation, promoting cartilage regeneration, and preventing chondrocyte apoptosis, MSC-Exos may serve as a viable and scalable alternative to current MSC-based therapies for this widespread degenerative disease.
Keywords: MSC-derived exosomes; anti-inflammation; ex vivo OA model; osteoarthritis; regenerative medicine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Injection of human umbilical cord mesenchymal stem cells exosomes for the treatment of knee osteoarthritis: from preclinical to clinical research.J Transl Med. 2025 Jun 11;23(1):641. doi: 10.1186/s12967-025-06623-y. J Transl Med. 2025. PMID: 40500748 Free PMC article. Clinical Trial.
-
Exosomes-Shuttled lncRNA SNHG7 by Bone Marrow Mesenchymal Stem Cells Alleviates Osteoarthritis Through Targeting miR-485-5p/FSP1 Axis-Mediated Chondrocytes Ferroptosis and Inflammation.Tissue Eng Regen Med. 2024 Dec;21(8):1203-1216. doi: 10.1007/s13770-024-00668-8. Epub 2024 Oct 3. Tissue Eng Regen Med. 2024. PMID: 39363054
-
Mesenchymal stem cell-derived exosomes for the treatment of knee osteoarthritis: a systematic review and meta-analysis based on rat model.Front Pharmacol. 2025 Jun 2;16:1588841. doi: 10.3389/fphar.2025.1588841. eCollection 2025. Front Pharmacol. 2025. PMID: 40529485 Free PMC article.
-
Exosomes Extracted from Human Umbilical Cord MSCs Contribute to Osteoarthritic Cartilage and Chondrocytes Repair Through Enhancing Autophagy While Suppressing the Wnt/β-Catenin Pathway.Tissue Eng Regen Med. 2025 Jul;22(5):719-733. doi: 10.1007/s13770-025-00716-x. Epub 2025 Apr 15. Tissue Eng Regen Med. 2025. PMID: 40232653
-
Mesenchymal stromal cells-derived extracellular vesicles in cartilage regeneration: potential and limitations.Stem Cell Res Ther. 2025 Jan 23;16(1):11. doi: 10.1186/s13287-025-04135-6. Stem Cell Res Ther. 2025. PMID: 39849578 Free PMC article.
References
-
- Hong J.W., Noh J.H., Kim D.-J. The prevalence of and demographic factors associated with radiographic knee osteoarthritis in Korean adults aged ≥ 50 years: The 2010–2013 Korea National Health and Nutrition Examination Survey. PLoS ONE. 2020;15:e0230613. doi: 10.1371/journal.pone.0230613. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

