Comparative Diagnostic Performance of Copeptin After Hypertonic Saline Infusion Versus Water Deprivation Test in Pediatric Patients with Polyuria-Polydipsia Syndrome
- PMID: 40564913
- PMCID: PMC12193309
- DOI: 10.3390/ijms26125449
Comparative Diagnostic Performance of Copeptin After Hypertonic Saline Infusion Versus Water Deprivation Test in Pediatric Patients with Polyuria-Polydipsia Syndrome
Abstract
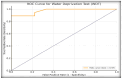
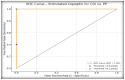
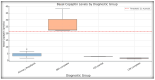
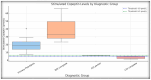
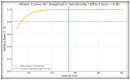
Differentiating central diabetes insipidus (CDI), nephrogenic diabetes insipidus (NDI), and primary polydipsia (PP) in pediatric patients with polyuria-polydipsia syndrome (PPS) remains a clinical challenge. The water deprivation test (WDT) is the traditional gold standard; however, it is time-consuming, burdensome, and prone to equivocal results. Stimulated copeptin, a surrogate marker of vasopressin, has emerged as a promising diagnostic alternative. We conducted a prospective, observational, cross-sectional study involving 27 pediatric patients (ages 2-17) presenting with PPS. Each patient underwent a WDT with desmopressin and hypertonic saline infusion (3% NaCl) for stimulated copeptin testing. Diagnostic accuracy was assessed using clinical diagnoses as a reference. The WDT showed high accuracy with an area under the curve (AUC) of 0.97, and there was an increased optimal threshold of ≥14% urine osmolality after desmopressin acetate (1-deamino-8-D-arginine vasopressin, DDAVP) administration (sensitivity 88.9%, specificity 100%). Stimulated copeptin at a threshold of <6.5 pmol/L demonstrated 100% sensitivity and specificity (AUC = 1.00) for CDI versus PP. Basal copeptin ≥21.4 pmol/L accurately identified all NDI cases. The agreement between the WDT and copeptin was low (κ = 0.06, McNemar p = 0.021), suggesting that copeptin has greater specificity, particularly for borderline or partial CDI. These results support the use of stimulated copeptin as a first-line diagnostic tool in pediatric PPS, offering improved objectivity, tolerability, and diagnostic clarity compared with the WDT. Basal copeptin also demonstrated excellent performance in rapid noninvasive NDI identification.
Keywords: arginin–vasopresin; central diabetes insipidus; copeptin; nephrogenic diabetes insipidus; primary polydipsia; water deprivation test.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
A post-hoc internal validation of arginine-stimulated copeptin cut-offs for diagnosing AVP deficiency (central diabetes insipidus).Pituitary. 2025 Apr 26;28(3):53. doi: 10.1007/s11102-025-01523-2. Pituitary. 2025. PMID: 40281371 Free PMC article.
-
Diagnostic Utility of Copeptin in Pediatric Patients with Polyuria-Polydipsia Syndrome: A Systematic Review and Meta-Analysis.Int J Mol Sci. 2024 Oct 5;25(19):10743. doi: 10.3390/ijms251910743. Int J Mol Sci. 2024. PMID: 39409072 Free PMC article.
-
The changing landscape in the evaluation of hypotonic polyuria in children and adolescents: the role of the new copeptin stimulation tests.J Pediatr Endocrinol Metab. 2025 May 23;38(7):703-717. doi: 10.1515/jpem-2025-0046. Print 2025 Jul 28. J Pediatr Endocrinol Metab. 2025. PMID: 40420740 Review.
-
Arginine or Hypertonic Saline-Stimulated Copeptin to Diagnose AVP Deficiency.N Engl J Med. 2023 Nov 16;389(20):1877-1887. doi: 10.1056/NEJMoa2306263. N Engl J Med. 2023. PMID: 37966286 Clinical Trial.
-
A COMBINED OUTPATIENT AND INPATIENT OVERNIGHT WATER DEPRIVATION TEST IS EFFECTIVE AND SAFE IN DIAGNOSING PATIENTS WITH POLYURIA-POLYDIPSIA SYNDROME.Endocr Pract. 2018 Nov;24(11):963-972. doi: 10.4158/EP-2018-0238. Epub 2018 Aug 14. Endocr Pract. 2018. PMID: 30106630
References
-
- Winzeler B., Cesana-Nigro N., Refardt J., Vogt D.R., Imber C., Morin B., Popovic M., Steinmetz M., Sailer C.O., Szinnai G., et al. Arginine-Stimulated Copeptin Measurements in the Differential Diagnosis of Diabetes Insipidus: A Prospective Diagnostic Study. Lancet. 2019;394:587–595. doi: 10.1016/S0140-6736(19)31255-3. - DOI - PubMed
-
- Atila C., Loughrey P.B., Garrahy A., Winzeler B., Refardt J., Gildroy P., Hamza M., Pal A., Verbalis J.G., Thompson C.J., et al. Central Diabetes Insipidus from a Patient’s Perspective: Management, Psychological Co-Morbidities, and Renaming of the Condition: Results from an International Web-Based Survey. Lancet Diabetes Endocrinol. 2022;10:700–709. doi: 10.1016/S2213-8587(22)00219-4. - DOI - PubMed
-
- Gubbi S., Hannah-Shmouni F., Koch C.A., Verbalis J.G. Diagnostic Testing for Diabetes Insipidus. In: Feingold K.R., Ahmed S.F., Anawalt B., Blackman M.R., Boyce A., Chrousos G., Corpas E., de Herder W.W., Dhatariya K., Dungan K., et al., editors. Endotext [Internet] MDText.com, Inc.; South Dartmouth, MA, USA: 2000.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

