Electrospun DegraPol Tube Delivering Stem Cell/Tenocyte Co-Culture-Derived Secretome to Transected Rabbit Achilles Tendon-In Vitro and In Vivo Evaluation
- PMID: 40564922
- PMCID: PMC12192585
- DOI: 10.3390/ijms26125457
Electrospun DegraPol Tube Delivering Stem Cell/Tenocyte Co-Culture-Derived Secretome to Transected Rabbit Achilles Tendon-In Vitro and In Vivo Evaluation
Abstract
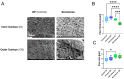
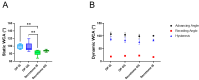
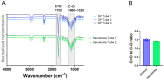
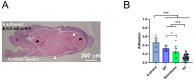
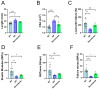
Tendon ruptures have recently reached incidences of 18-35 cases/100,000 and often lead to adhesion formation during healing. Furthermore, scar formation may result in inferior biomechanics and often leads to re-ruptures. To address these problems, we cultivated rabbit adipose-derived stem cells in a co-culture with rabbit Achilles tenocytes and harvested their secretome. Following a cell-free approach, we incorporated such secretome into an electrospun tube via emulsion electrospinning. These novel implants were characterized by SEM, the WCA, and FTIR. Then, they were implanted in the rabbit Achilles tendon full transection model with an additional injection of secretome, and the adhesion extent as well as the biomechanics of extracted tendons were assessed three weeks postoperatively. The fiber thickness was around 3-5 μm, the pore size 11-13 μm, and the tube wall thickness approximately 265 μm. The WCA indicated slightly hydrophilic surfaces in the secretome-containing layer, with values of 80-90°. In vivo experiments revealed a significant reduction in adhesion formation (-22%) when secretome-treated tendons were compared to DegraPol® (DP) tube-treated tendons (no secretome). Furthermore, the cross-sectional area was significantly smaller in secretome-treated tendons compared to DP tube-treated ones (-32%). The peak load and stiffness of secretome-treated tendons were not significantly different from native tendons, while tendons treated with pure DP tubes exhibited significantly lower values. We concluded that secretome treatment supports tendon healing, with anti-adhesion effects and improved biomechanics at 3 weeks, making this approach interesting for clinical application.
Keywords: adhesion; biomechanics; mesenchymal stem cells; rabbit; secretome; tendon healing; tenocytes.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Hoeffner R., Agergaard A.S., Svensson R.B., Cullum C., Mikkelsen R.K., Konradsen L., Krogsgaard M., Boesen M., Kjaer M., Magnusson S.P. Tendon Elongation and Function After Delayed or Standard Loading of Surgically Repaired Achilles Tendon Ruptures: A Randomized Controlled Trial. Am. J. Sports Med. 2024;52:1022–1031. doi: 10.1177/03635465241227178. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

