Uridine, a Therapeutic Nucleoside, Exacerbates Alcoholic Liver Disease via SRC Kinase Activation: A Network Toxicology and Molecular Dynamics Perspective
- PMID: 40564937
- PMCID: PMC12193447
- DOI: 10.3390/ijms26125473
Uridine, a Therapeutic Nucleoside, Exacerbates Alcoholic Liver Disease via SRC Kinase Activation: A Network Toxicology and Molecular Dynamics Perspective
Abstract
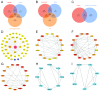
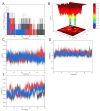
This study looked into the underlying mechanisms and causal relationship between alcoholic liver disease (ALD) and the blood metabolite uridine using a variety of analytical methods, such as Mendelian randomization and molecular dynamics simulations. We discovered uridine to be a possible hepatotoxic agent aggravating ALD by using Mendelian randomization (MR) analysis with genome-wide association study (GWAS) data from 1416 ALD cases and 217,376 controls, as well as with 1091 blood metabolites and 309 metabolite concentration ratios as exposure factors. According to network toxicology analysis, uridine interacts with important targets such as SRC, FYN, LYN, ADRB2, and GSK3B. The single-cell RNA sequencing analysis of ALD tissues revealed that SRC was upregulated in hepatocytes and activated hepatic stellate cells. Subsequently, we determined the stable binding between uridine and SRC through molecular docking and molecular dynamics simulation (RMSD = 1.5 ± 0.3 Å, binding energy < -5.0 kcal/mol). These targets were connected to tyrosine kinase activity, metabolic reprogramming, and GPCR signaling by Gene Ontology (GO) and KEGG studies. These findings elucidate uridine's role in ALD progression via immunometabolic pathways, offering novel therapeutic targets for precision intervention. These findings highlight the necessity of systems biology frameworks in drug safety evaluation, particularly for metabolites with dual therapeutic and toxicological roles.
Keywords: alcoholic liver disease; mendelian randomization; molecular dynamics simulation; network toxicology; single-cell RNA sequencing.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Song H., Zhou H., Yang S., He C. Combining mendelian randomization analysis and network toxicology strategy to identify causality and underlying mechanisms of environmental pollutants with glioblastoma: A study of Methyl-4-hydroxybenzoate. Ecotoxicol. Environ. Saf. 2024;287:117311. doi: 10.1016/j.ecoenv.2024.117311. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

