Structural Insights into the ADCC Mechanism and Resistance of Mogamulizumab, a First-in-Class Anti-CCR4 Therapy for Cutaneous T Cell Lymphoma
- PMID: 40564964
- PMCID: PMC12193575
- DOI: 10.3390/ijms26125500
Structural Insights into the ADCC Mechanism and Resistance of Mogamulizumab, a First-in-Class Anti-CCR4 Therapy for Cutaneous T Cell Lymphoma
Abstract
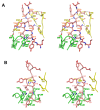
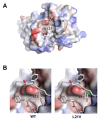
Mogamulizumab is a humanized monoclonal antibody that targets C-C chemokine receptor 4 (CCR4) present on certain T cells in lymphomas and leukemias. This antibody-based therapy has demonstrated efficacy in treating various cutaneous T cell lymphomas (CTCLs), including mycosis fungoides and Sézary syndrome, through the depletion of CCR4-expressing T cells by antibody-dependent cellular cytotoxicity (ADCC). However, the precise epitope and binding mode of mogamulizumab responsible for its augmented ADCC activity remain undisclosed. Here, X-ray crystallographic studies of mogamulizumab in complex with a 28-residue N-terminal peptide indicated that SIYSNYYLYES (residues 14-24) would constitute the antibody epitope. Another high-resolution structure, using a short core peptide of these 11 residues, has elucidated unambiguous electron density for the bound peptide, confirming consistent binding for both peptides. This linear epitope is located in the membrane-proximal region of CCR4, facilitating the Fc-mediated effector functions, including ADCC. The structures also provide insights into the molecular basis for the resistance of the CCR4 L21V variant to mogamulizumab, which is due to a lack of structural complementarity with mogamulizumab binding. Understanding the structural basis for the mechanism of action of mogamulizumab is crucial for optimizing anti-CCR4 therapeutics to improve treatment outcomes for patients with these challenging diseases.
Keywords: C-C chemokine receptor 4 (CCR4); X-ray structure; antibody; antibody-dependent cellular cytotoxicity (ADCC); cutaneous T cell lymphoma (CTCL); drug resistance; mogamulizumab.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

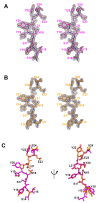

Similar articles
-
Evaluating mogamulizumab in the treatment of primary cutaneous T-cell lymphoma.Immunotherapy. 2025 Jun;17(8):551-559. doi: 10.1080/1750743X.2025.2520153. Epub 2025 Jun 24. Immunotherapy. 2025. PMID: 40552425 Review.
-
Therapeutic advances for cutaneous T-cell lymphoma.Br J Dermatol. 2025 Jun 20;193(1):11-15. doi: 10.1093/bjd/ljaf105. Br J Dermatol. 2025. PMID: 40131851 Review.
-
Practical recommendations for therapy and monitoring of mogamulizumab patients in Germany.J Dtsch Dermatol Ges. 2025 Mar;23(3):341-354. doi: 10.1111/ddg.15639. Epub 2024 Dec 26. J Dtsch Dermatol Ges. 2025. PMID: 39723687 Free PMC article. Review.
-
Mogamulizumab for Previously Treated Mycosis Fungoides and Sézary Syndrome: An Evidence Review Group Perspective of a NICE Single Technology Appraisal.Pharmacoeconomics. 2022 May;40(5):509-518. doi: 10.1007/s40273-021-01098-3. Epub 2021 Oct 19. Pharmacoeconomics. 2022. PMID: 34664200 Free PMC article.
-
Cutaneous T-Cell Lymphoma: Optimizing Care in Patients Receiving Anti-CCR4 Monoclonal Antibody Mogamulizumab.Clin J Oncol Nurs. 2019 Aug 1;23(4):E73-E80. doi: 10.1188/19.CJON.E73-E80. Clin J Oncol Nurs. 2019. PMID: 31322628 Review.
References
-
- Agar N.S., Wedgeworth E., Crichton S., Mitchell T.J., Cox M., Ferreira S., Robson A., Calonje E., Stefanato C.M., Wain E.M., et al. Survival Outcomes and prognostic Factors in Mycosis Fungoides/Sézary Syndrome: Validation of the Revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer Staging Proposal. J. Clin. Oncol. 2010;28:4730–4739. doi: 10.1200/JCO.2009.27.7665. - DOI - PubMed
-
- Trautinger F., Eder J., Assaf C., Bagot M., Cozzio A., Dummer R., Gniadecki R., Klemke C.-D., Ortiz-Romero P.L., Papadavid E., et al. European Organisation for Research and Treatment of Cancer consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome—Update 2017. Eur. J. Cancer. 2017;77:57–74. doi: 10.1016/j.ejca.2017.02.027. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

