Mitochondrial Transfer from Human Platelets to Rat Dental Pulp-Derived Fibroblasts in the 2D In Vitro System: Additional Implication in PRP Therapy
- PMID: 40564967
- PMCID: PMC12192669
- DOI: 10.3390/ijms26125504
Mitochondrial Transfer from Human Platelets to Rat Dental Pulp-Derived Fibroblasts in the 2D In Vitro System: Additional Implication in PRP Therapy
Abstract
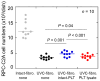
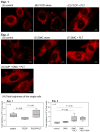
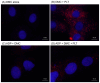
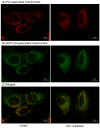
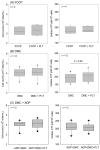
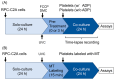
Platelet mitochondria have recently been increasingly considered "co-principal" along with platelet growth factors to facilitate tissue regeneration in platelet-rich plasma therapy cooperatively. To develop a convenient method to test this potential, we examined mitochondrial transfer using a simple two-dimensional culture system. Living human platelets were prepared from PRP obtained from 12 non-smoking healthy male adults (age: 28-63 years) and suspended in medium. Platelet lysates were prepared from sonicated platelet suspensions in PBS. After treatment with ultraviolet-C irradiation, a mitochondrial respiration inhibitor, or a synchronized culture reagent, rat dental pulp-derived fibroblasts (RPC-C2A) were co-cultured with platelets or platelet lysates for 24 h. Mitochondrial transfer was evaluated by visualization using a fluorescent dye for mitochondria or an antibody against human mitochondria. Ultraviolet-C-irradiated cells substantially lost their viability, and treatment with living platelets, but not platelet lysates, significantly rescued the damaged fibroblasts. Fibroblast mitochondria appeared to increase after co-culture with resting platelets. Although more microparticles existed around the platelets on the fibroblast surface, the activated platelets did not show significant increases in any parameters of mitochondrial transfer. This simple co-culture system demonstrated mitochondrial transfer between xenogeneic cells, and this phenomenon should be considered as an additional implication in PRP therapy.
Keywords: fibroblasts; in vitro; mitochondria; platelet-rich plasma; platelets; transfer.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Knightly N., Lee C., O’Brien L., Qayyum T., Hurley C., Kelly J. Role for platelet rich plasma as an adjuvant therapy in wound healing and burns. Eur. J. Plast. Surg. 2023;46:465–474. doi: 10.1007/s00238-023-02050-8. - DOI
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

