Potential of Pandan Root and Teak Leaf Extracts in Managing Maternal Hyperglycemia During Pregnancy: Comparative Efficacy and Mechanistic Insights
- PMID: 40564970
- PMCID: PMC12193165
- DOI: 10.3390/ijms26125506
Potential of Pandan Root and Teak Leaf Extracts in Managing Maternal Hyperglycemia During Pregnancy: Comparative Efficacy and Mechanistic Insights
Abstract
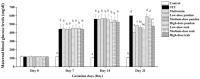
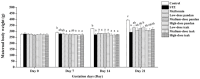
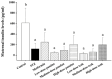
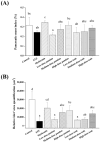
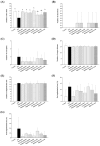
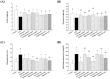
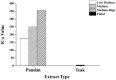
Maternal hyperglycemia during pregnancy poses significant health risks to both mother and fetus. Although gestational diabetes mellitus (GDM) is mainly characterized by insulin resistance, severe hyperglycemia may also result from impaired pancreatic function. This study evaluates the therapeutic potential of pandan (Pandanus amaryllifolius) root and teak (Tectona grandis) leaf extracts in managing streptozotocin (STZ)-induced maternal hyperglycemia in pregnant rats, compared to metformin. Methods: Pregnant rats were administered STZ (60 mg/kg) on gestation day 5. Treatments with metformin (300 mg/kg), pandan extract (low, medium, high doses), and teak extract (low, medium, high doses) were given from gestation day 7 to 21. The key parameters included the maternal blood glucose, insulin levels, pancreatic morphology, fetal and placental outcomes, and gas chromatography/mass spectrometry (GC/MS) phytochemical profiling. GC/MS analysis identified 2,3-butanediol and propanoic acid derivatives as major compounds in pandan, while teak contained catavic acid and methyl copalate. The high-dose pandan extract significantly reduced the maternal blood glucose (p < 0.05), improved the insulin levels and pancreatic mass index, and increased the number of live fetuses, with effects comparable to metformin. The teak extract showed milder improvements. The pandan extract demonstrated dose-dependent antidiabetic potential in this STZ-induced model. Future studies should evaluate these effects in insulin-resistance-based GDM models.
Keywords: gestational diabetes mellitus; maternal hyperglycemia; pandan root extract; streptozotocin-induced diabetes; teak leaf extract.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Antidiabetic Evaluation of Kigelia pinnata Root Bark Extract in Streptozotocin-Induced Type-2 Diabetes Model of Rats.Assay Drug Dev Technol. 2024 May-Jun;22(4):169-180. doi: 10.1089/adt.2023.104. Epub 2024 Mar 28. Assay Drug Dev Technol. 2024. PMID: 38546423
-
Antidiabetogenic action of Morus rubra L. leaf extract in streptozotocin-induced diabetic rats.J Pharm Pharmacol. 2010 Feb;62(2):247-55. doi: 10.1211/jpp.62.02.0013. J Pharm Pharmacol. 2010. PMID: 20487205
-
Oral anti-diabetic agents for women with established diabetes/impaired glucose tolerance or previous gestational diabetes planning pregnancy, or pregnant women with pre-existing diabetes.Cochrane Database Syst Rev. 2017 Oct 18;10(10):CD007724. doi: 10.1002/14651858.CD007724.pub3. Cochrane Database Syst Rev. 2017. PMID: 29045765 Free PMC article.
-
Metformin for women who are overweight or obese during pregnancy for improving maternal and infant outcomes.Cochrane Database Syst Rev. 2018 Jul 24;7(7):CD010564. doi: 10.1002/14651858.CD010564.pub2. Cochrane Database Syst Rev. 2018. PMID: 30039871 Free PMC article.
-
Unlocking the therapeutic potential of Catharanthus roseus leaves via in-vitro, in-vivo, and in-silico study.Sci Rep. 2025 Jul 17;15(1):25909. doi: 10.1038/s41598-025-96643-x. Sci Rep. 2025. PMID: 40676081 Free PMC article.
References
-
- Picón-César M.J., Molina-Vega M., Suárez-Arana M., González-Mesa E., Sola-Moyano A.P., Roldan-López R., Romero-Narbona F., Olveira G., Tinahones F.J., González-Romero S. Metformin for gestational diabetes study: Metformin vs insulin in gestational diabetes: Glycemic control and obstetrical and perinatal outcomes: Randomized prospective trial. Am. J. Obstet. Gynecol. 2021;225:517.e1–517.e17. doi: 10.1016/j.ajog.2021.04.229. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

